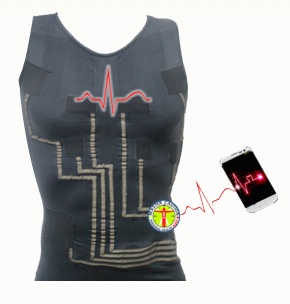
 Tel Aviv, Israel-based HealthWatch is working on another entry for the still emerging smart textile field. At the American Telemedicine Association conference in Baltimore, Maryland, the startup previewed its hWear line of tshirts with interwoven ECG sensors, allowing the shirt to function as a 3 to 15 lead ECG.
Tel Aviv, Israel-based HealthWatch is working on another entry for the still emerging smart textile field. At the American Telemedicine Association conference in Baltimore, Maryland, the startup previewed its hWear line of tshirts with interwoven ECG sensors, allowing the shirt to function as a 3 to 15 lead ECG.
"A cardiologist will not formally diagnose a heart attack until you’ve taken a 12-lead ECG," Dr. Dov Rubin, VP of Marketing and Business Development at HealthWatch told MobiHealthNews. "So if you call up your cardiologist and say 'Doc, I’ve got heart pain,' ... That really doesn’t help the cardiologist. They say 'Come into the office, I’ll set you up with a 12-lead ECG'. You can imagine time is of the essence. If you’re wearing a garment with the 12 leads, it will send it instantly, so the cardiologist, based on the last 10 minutes of your ECG, can see exactly what’s going on. Based on that, he can diagnose a heart attack. He can get you to the hospital, get a stent, do what needs to be done."
The hWear tshirts are machine washable and can be tumble-dried. There is an electronic component that hooks into the shirt -- a box that can store up to 70 hours of data or transmit data wirelessly to Android smartphones -- which must be removed before washing. Rubin said the shirts can be washed about 50 times without deteriorating.
"How do you design a garment with tension in certain key places, so the electrodes sit right, but is still comfortable for wearing and insulating?" Rubin said, explaining some of the challenges in creating the garments. "How do you get over the issue of movement? Those are some of the breakthroughs here. We really have a very clean signal, even in motion."
Rubin said that initially the company wants to get the tshirts in use by hospitals for situations where longer-term monitoring than a Holter can provide is required. They want to establish validity in a clinical setting before launching a wellness-focused, direct-to-consumer product. The company is also interested in the telemonitoring space, where the tshirt could automatically alert doctors if it detected an arrhythmia or a heart attack.
Currently, the garment itself is registered with the FDA as a Class I device, but the company is seeking 510(k) clearance for the complete system. HealthWatch estimates the cost of the tshirts will be around $200 to the end user. In the future, the company is also looking at designing a version for pregnant women that could be used for fetal ECG monitoring.
A lot of early stage companies are focused on garments with built-in sensors. Most notably, OMsignal, which raised $1 million last year, is working on an undershirt that has sensors woven into the fabric. The shirt captures ECG, activity, breathing patterns and “emotive” states on a continuous basis and presents that data to the wearer via an app on their mobile device. While the shirt can track ECG, the app doesn’t show it in that form because OMsignal itself isn’t looking to make the shirt an FDA regulated medical device.
Initially, though, HealthWatch is more likely to compete with offerings like iRhythm's ZIO Patch, an adhesive patch sensor which Aetna recently began reimbursing for as a Holter monitor replacement, after a Scripps study found it comparably effective.













