 In the last two months, the FDA has cleared a number of clinically-focused mobile health devices. Some are first-time clearances for companies that have been waiting on their 510(k) for a while, including a few MobiHealthNews has been keeping an eye on over the years. Others are incremental clearances for slight hardware or algorithm updates. Here's five mobile health clearances that came through in April or May.
In the last two months, the FDA has cleared a number of clinically-focused mobile health devices. Some are first-time clearances for companies that have been waiting on their 510(k) for a while, including a few MobiHealthNews has been keeping an eye on over the years. Others are incremental clearances for slight hardware or algorithm updates. Here's five mobile health clearances that came through in April or May.
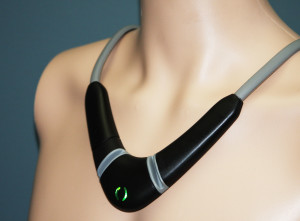
toSense's CoVa monitoring system: We wrote about the CoVa monitoring system last year when the company was making the rounds at HIMSS with its necklace for clinical use that can track a number of vital signs including thoracic fluid levels, an early predictor of congestive heart failure that isn’t currently monitored by most connected sensors. ToSense, formerly known as Perminova, has now received FDA 510(k) clearance for its device, which is intended to be worn for a few minutes a day by patients managing their care at home. The startup lists Scripps Health cardiologist Eric Topol as an advisor.
“Heart failure is the most important cause of hospital admission and readmission in the United States,” Topol said in a statement. “This innovative necklace technology to track chest fluid status and key heart parameters has promise for use in patients with heart failure.”
Jintronix: Jintronix is one of a handful of companies working on Microsoft Kinect software for physical therapy rehabilitation. At the time of MobiHealthNews's Kinect the Docs report, none of those companies had yet secured FDA 510(k) clearance as a medical device, though several were working on it.
Jintronix offers providers specially designed, clinically tested video games that both allow patients to exercise and collect data about their exercises to send to an online portal. On the portal, physicians can make changes to the difficulty and range of motion in the games as well as preparing for their patients’ next visit with the information about their movement that Microsoft Kinect tracked. The clearance is prescription only, so it will require that the software be used with the guidance of a physician.
InvisionHeart ECG Device: This is the first FDA clearance for InvisionHeart, which offers a mobile 12-lead ECG that can be accessed via a secure, browser-based online system. The company believes having the data in the cloud will make it easier for providers to access the data more quickly, leading to more timely interventions for heart patients. The company hopes to launch the device for hospital customers in the third quarter.
“The InvisionECG system will offer significant advancement in cardiac care for patients and healthcare providers in a wide variety of care settings," Melanie Varin, Executive Vice President of Sales and Marketing, said in a statement. "Initial interest has been strong from a broad array of potential users, especially those challenged to find a high-quality, low-cost solution that is secure and fully electronic. In addition to hospitals, we’ve detected genuine enthusiasm from the rapidly growing markets for home health and ambulatory care as well as other specialty markets.”
Sotera Wireless's ViSi Mobile system: ViSi Mobile got FDA clearance way back in 2012, but continues to get new clearances as it updates its algorithm. The latest clearance adds better posture and gait tracking -- and fall detection -- to the ambulatory remote patient monitoring system.
"The modifications were to add the following posture alarm features:" -- the summary document reads -- "(1) undesirable posture technical alarm,(2) patient immobility technical alarm, and (3) patient fall alarm. The last modification (4) allows the system to display if the patient is walking along with the already existing ability to display if a patient is upright, reclined or lying-down. This modification is purely a visual icon and has no associated alarms. All four modifications were implemented to provide clinicians with information that will increase patient safety."
Vitaphone Post-Event Recorder: Vitaphone has been doing mobile ECG monitoring since at least 2010, using a 12-lead ECG and sending data via Bluetooth to a satellite phone to a patient's caregiver. The new clearance is a small change from their existing, cleared device: the new version will provide three ECG recording channels rather than just one.

















