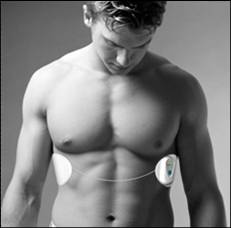
 Ireland-based Intelesens received FDA 510(k) clearance last month for its Aingeal wearable wireless hospital monitor, which passively monitors various vital signs.
Ireland-based Intelesens received FDA 510(k) clearance last month for its Aingeal wearable wireless hospital monitor, which passively monitors various vital signs.
According to the company, Aingeal differentiates itself from competitors in that it "measures the patient's ECG, heart rate, temperature and motion and sends that information wirelessly so that it is immediately and easily accessible by nursing and other medical staff."
Aingeal touts its device's ability to measure a patient's respiration rate. (Zephyr's BioHarness also measures breathing rate.) Aingeal's Intelesens offering, however, is more reminiscent of Sotera Wireless' ViSi Mobile system, which is also intended for use as a wireless vital signs monitoring system within care facilities.
In a press release, Intelesens CEO Michael Caulfield said "This has been a tremendous result following many months of meticulous testing and data gathering by the engineering and quality teams in Intelesens, who had to work very closely with the US regulatory authorities."
"Aingeal has been designed so that it can be used to monitor those patients who are moving around the hospital as well as those who are confined to beds. Typically only 40 percent of hospital beds are monitored -- this technology, for the first time, opens up the opportunity to provide low cost, ubiquitous vital signs monitoring for all patients within a hospital," Caulfield.
Clinical trials for the device took place at Massachusetts General Hospital last year ahead of the FDA clearance. Aingeal received its European CE class 2 approval last year. FDA clearances in 2011 already include Mobisante's smartphone ultrasound, iHealth's blood pressure cuff, Abbott's mobile blood analyzer, Mobile MIM's radiology app, and most recently, the Withings blood pressure cuff.


















