 While AirStrip just announced that it had secured a CE Mark for its suite of mobile medical applications last week, it may have not been the very first medical app to secure a CE Mark and be publicly available in the UK market. Another app, created in cooperation with a team of UK-based plastic surgeons, apparently carries that distinction.
While AirStrip just announced that it had secured a CE Mark for its suite of mobile medical applications last week, it may have not been the very first medical app to secure a CE Mark and be publicly available in the UK market. Another app, created in cooperation with a team of UK-based plastic surgeons, apparently carries that distinction.
According to D4, a non-profit organization in the UK that equips physicians and nurses with communications devices, the very first mobile medical app to be classified as a medical device in the UK recently registered as a Class I medical device with that country's Medicines and Healthcare products Regulatory Agency (MHRA). The MHRA recognizes the EU's Medical Device Directive's classification system, which makes use of the CE Mark to designates a device is registered and in compliance with the regulatory system.
"It is understood that to date only one app that is publically available for download has been registered as a medical device with the MHRA in the UK," D4 writes in a new report focused on the regulation of mobile medical apps in the UK.
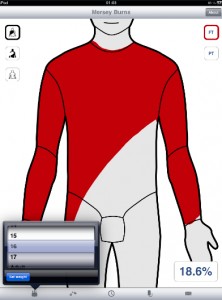
The app is called Mersey Burns and it's currently available for all iOS devices. The Mersey Plastic Surgery Unit helped to develop the app, which aims to help doctors more easily calculate the percentage of a patient's body surface area that is burned and, in turn, to better calculate the amount of fluid to be administered in the 24-hour period that follows the burn injury. A small study found that those using the app had less variance among estimates for total body surface area that was affected by the burn, while those using paper based assessment tools had greater variance.
D4 included a short quote from the MHRA that provides some insight into the agency's thinking around the regulation of medical apps: "Only after a product meets the definition of a medical device does it get classified according to risk, the risk classification then determines the compliance requirements. There is clearly a full range of application from the simple non-clinical to potentially complex ones for medical use," the agency told D4. "As an example, if the application is intended to carry out further calculations, enhancements or interpretations of entered/captured patient data, we consider that it will be a Medical Device. If it carries out complex calculations, which replaces the clinician’s own calculation and which will therefore be relied upon, then it will certainly be considered a Medical Device."
While the report is largely positioned as a UK-focused study, its recommendations may have wider appeal. D4 explains: "Within the UK, the MHRA is responsible as the Competent Authority under the Medical Device Directive, and provides guidance to device manufacturers. However at present there is no central European register of registered medical devices. Each Competent Authority manages its own register, and a manufacturer needs only to register in one member state to place its device on the market across the EU."
When it comes to the US market: MobiHealthNews broke the news last July that the FDA had published a set of draft guidelines for how it suggested it might regulate mobile medical apps moving forward. Following a 90-day comment period that ended last fall, the FDA is now working to publish a final set of guidelines sometime this year. AirStrip's OB app was the first iPhone medical app to secure FDA clearance in the US.
Be sure to read the full UK-focused D4 report for free here (PDF). More in D4's press release below:
PRESS RELEASE: UK charity draws attention to the regulation of health apps and publishes guidance document to help health professionals, organisations, patients and industry
10 January 2012, London – A new app, Mersey Burns, has been released on to the market that represents a first in the UK – it has been registered with the MHRA as a Class I medical device as per the EU Medical Device Directive. To coincide with this, d4 have simultaneously published a new guidance document to help draw further attention to the issue of health app regulation and provide practical guidance to both users and manufacturers of apps for the healthcare market.
Health professionals make considerable use of mobile phones during their working day, as do their patients. As the popularity of running software applications on mobile devices continues to increase, we anticipate that the use of apps to aid medical diagnosis and treatment will gain in popularity with a corresponding increase in risk to the general public. Specific regulations that accompany this nascent technology are in their infancy, but should not be ignored.
For all stakeholders concerned, it is in our collective interest to support responsible use of this new technology. It will take one high profile failing to cause a loss of trust that can take months, if not years, to rebuild. In their guidance document, Regulation of health apps: a practical guide, d4 make the following recommendations:
Health professionals should carefully consider the risks when using apps to determine a patient’s care.
Developers should test their apps thoroughly and maintain adequate technical documentation to evidence this.
Publishers should ensure compliance with the necessary regulations before releasing apps on to the market.
Organisations should investigate ways to manage the use of apps by their employees, and put in place mechanisms to identify those apps that are deemed fit for professional use.
Patients should examine carefully the source of the apps they use to manage their health. Within Europe, health apps that influence a patient’s treatment should carry the CE mark to demonstrate their conformity with the appropriate regulation.
“mHealth is a new industry and the regulatory environment is evolving,” said James Sherwin-Smith, CEO of d4. ”Regulators are necessary to safeguard the public and uphold confidence in markets that would otherwise be open to potential abuse. But regulations also need to support, and not stifle, innovation. The regulatory issues that surround health apps are complex and open to interpretation. We hope that this guide provides a useful steer for individuals and organisations alike.”
ENDS
Notes for editors:
d4 is a non-profit organisation with registered charity status in England and Wales. Founded on the belief that better communication means better care, d4 aims to improve patient care by placing modern technology in the hands of doctors, nurses and other health care professionals. For more information please contact James Sherwin-Smith on 0845 686 3434 or visit the d4 website at http://www.d4.org.uk
















