 A startup company is looking to raise $650,000 on Indiegogo to test and eventually bring to market a wireless sensor meant to aid in the early detection of breast cancer and the tracking of patients being treated for the disease that kills 40,000 U.S. women each year.
A startup company is looking to raise $650,000 on Indiegogo to test and eventually bring to market a wireless sensor meant to aid in the early detection of breast cancer and the tracking of patients being treated for the disease that kills 40,000 U.S. women each year.
Eclipse Breast Health Technologies, of La Mesa, California, launched its crowdfunding campaign Monday and hopes to reach its goal by Oct. 3.
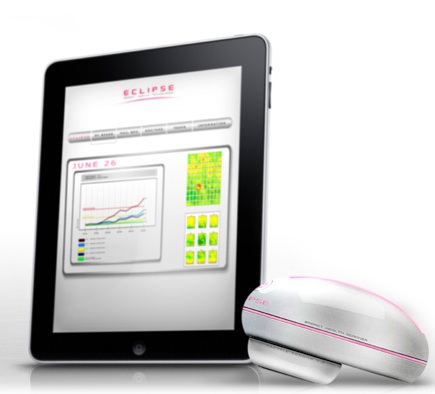
The system consists of a handheld imaging device paired with imaging software that tracks changes in breast density over time, and also incorporates an element of social networking. Eclipse says the device, which uses what the company calls "transphotonic" technology – combining low-energy photons and light sensors, without the need for radiation – is as much as five times as sensitive as the human hand. (In a video on the Indiegogo page, the company calls the light-based imaging "100 percent safe."
"It mimics the breast self-exam for women," said Eclipse founder and CEO Ken Wright, who helped develop similar technology for U.S Navy submarines to identify tiny objects in murky water.
The imaging device links wirelessly or by USB to a PC, smartphone or tablet, where a Web application creates a visual representation of the self-exam and helps quantify observations. Data is uploaded to patient accounts on a platform that calls Pink Cloud. From there, women can compare readings over time, share the information with their physicians or connect with others on Pink Cloud, which incorporates a social network of Eclipse users.
Wright said the prototype system is not for self-diagnosis, nor is it a substitute for traditional mammography. Instead, it is intended to improve the self-examination process and to help women track self-exams from month to month. "We are not trying to change any of the protocols," Wright told MobiHealthNews.
"It also can be a tool for physicians to monitor an area over time," Wright said. For example, the Eclipse imaging device might allow an oncologist to track a tumor's shrinking during a course of chemotherapy.
Wright did indicate, however, that this noninvasive way of detecting abnormalities "gives promise" for future, and the long-range plan is to develop something that could be used for diagnostic purposes.
For now, Eclipse seeks nearly two-thirds of a million dollars to move into a beta testing phase with about 1,000 women by the first quarter of 2014, with the intention of going to market by the end of next year, Wright said. (Those who contribute at least $199 are offered a chance to be part of the pilot.)
Testers will try the device on model breasts rather than their own bodies. "We don't want to cause any false alarms at this point," Wright explained. "We want to make sure we get this absolutely right."


















