 Yesterday, Apple announced its rumored health data aggregation platform, called HealthKit and a companion app called Health, at the company's annual World Wide Developer's Conference. The app will integrate with a variety of health and wellness apps currently on the market so that users can put all their data in one place. According to Apple senior vice president of Software Engineering Craig Federighi, this kind of integration can provide users with "a single comprehensive picture of your health situation.”
Yesterday, Apple announced its rumored health data aggregation platform, called HealthKit and a companion app called Health, at the company's annual World Wide Developer's Conference. The app will integrate with a variety of health and wellness apps currently on the market so that users can put all their data in one place. According to Apple senior vice president of Software Engineering Craig Federighi, this kind of integration can provide users with "a single comprehensive picture of your health situation.”
Apple has come a long way since its 2009 WWDC event when the company announced the iPhone could connect with medical devices, but so have other digital health startups and companies. The picture of patient generated health data has evolved significantly over the last five years. Along the way, accelerators were born that are dedicated specifically to digital health, the FDA published its final guidance on the regulation of mobile medical apps, and some larger companies including Intel and Facebook have acquired digital health startups.
MobiHealthNews has compiled a timeline of important events in the history of patient generated health data with a specific focus on the dedicated health devices that have made headlines over the years.
February 6, 2009: iTMP announces the launch of its SM Heart Link device, a "wireless bridge for biometrics" that connects off-the-shelf fitness sensors like heart rate straps to a user's iPhone. The device launches with a $155 pricepoint. Read More
March 17, 2009: At Apple's World Wide Developers Conference, just before showing off a connected blood pressure monitor, Scott Forstall, SVP of iPhone Software at Apple gushes: ”Now here’s a class [of services] that we think will be really interesting: medical devices.” Forstall explained that the new iPhone OS will allow application developers to sync medical devices like BP monitors via both Bluetooth and USB. “So imagine the possibilities,” Forstall continues. “We think this is profound.” An exec from Johnson & Johnson company LifeScan then shows off a prototype of a blood glucose meter that connects to the iPhone to feed data into a companion app. Read More
April 2, 2009: David Van Sickle, Robert Wood Johnson Foundation Health and Society Scholar in the Department of Population Health Sciences at the University of Wisconsin-Madison, reveals that he is developing a GPS add-on for asthmatics’ inhalers to map where and when environmental exposures cause asthma symptoms. The venture soon takes on the name Asthmapolis. Read More
April 8, 2009: CellScope, in its earliest stages at UC Berkeley as a smartphone-enabled microscope with remote diagnosis potential, wins a Vodafone Americas Foundation award. Read More
July 16, 2009: Entra Health Systems announces its MyGlucoHealth Clinical Point-of-Care System, which is a clinic-based diabetic testing for in-patient care environments. The offering includes a cloud-based interface called Clinical Point-of-Care that works with the MyGlucoHealth meter to upload blood glucose tests and data through Bluetooth or USB to tablet computer, PC or compatible PDA device. Read More
September 30, 2009: French technology company Withings announces the US availability of its WiFi Body Scale that automatically records the user’s body weight, lean and fat mass, and calculated body mass index (BMI) to his/her secure webpage and/or free Withings iPhone application, WiScale. Read More
December 30, 2009: Australian mobile operator Telstra inks a deal with Entra to bring its smartphone-enabled diabetes system to that country. Read More
Tools for patient generated health data in 2010
 April 7, 2010: Entra's MyGlucoHealth Diabetes App becomes available on Nokia's app store, the Ovi Store. Read More
April 7, 2010: Entra's MyGlucoHealth Diabetes App becomes available on Nokia's app store, the Ovi Store. Read More
July 9, 2010: Salem, MA-based Agamatrix believes that its WaveSense Jazz meter could become the first medical device to connect directly to Apple’s iOS platform, which includes iPad, iPhone and iPod touch. Why isn’t the WaveSense Jazz meter already sending data to the iPhone app? Agamatrix says its new USB download cable, which connects the meter to the iPhone, is currently pending FDA 510(k) review. Read More
September 21, 2010: French pharmaceutical company Sanofi Aventis announces that it has tapped Agamatrix to create blood glucose meter plug-in for Apple’s iPhone called iBGStar. Read More
October 7, 2010: BodyMedia announces plans for its new Bluetooth-enabled FIT Armband BW to transmit activity and other biometric data to BodyMedia users' smartphones for the first time.
December 15, 2010: FDA clears Zephyr's smartphone-enabled system: Zephyr’s OmniSense Mobile Smartphone based solution serves a broad array of applications from remote patient monitoring to employee safety. Read More
December 27, 2010: BodyMedia inks a deal with Jenny Craig that sees the weight loss company equipping some of its premium members with the wearable tracking company's FDA-cleared FIT device.
December 30, 2010: AliveCor's Dr. David Albert posts a video demo of his "iPhone ECG" device so a friend who couldn't make it an in-person demo at CES in Las Vegas the following month could check it out. The video goes viral and "Dr. Dave" ends up appearing on a number of talk shows and news programs as one of the breakout hits of CES that year. The device measures a single lead ECG from a custom iPhone case and transmits it via a mobile app on the user's iPhone. The case talks to the phone via a proprietary connectivity method. Video
Tools for patient generated health data in 2011
 January 3, 2011: BodyMedia announces a deal with Sprint that will add cellular connectivity to an upcoming version of its FIT Armband. The focus is always-on connectivity for the wearable that will stream data to BodyMedia's Android apps.
January 3, 2011: BodyMedia announces a deal with Sprint that will add cellular connectivity to an upcoming version of its FIT Armband. The focus is always-on connectivity for the wearable that will stream data to BodyMedia's Android apps.
January 4, 2011: iHealth Lab, a San Francisco-based subsidiary of Chinese medical company Andon Health, announces the iHealth Blood Pressure Monitoring System for iPhone. The offering includes a battery-powered hardware dock, blood pressure arm cuff and a corresponding app. The system works with the iPhone, iPad and iPod touch and looks to be the first FDA-cleared medical device to head to Apple Stores. Read More
January 5, 2011: France-based Withings announces the launch of its own blood pressure monitor for the iPhone. Read More
January 17, 2011: Dr. David Albert of AliveCor says that he has developed an Android version of its iPhoneECG device, too. Read More
January 20, 2011: The FDA clears the Withings iPhone blood pressure cuff as a Class II medical device. Read More
February 4, 2011: FDA clears the smartphone-enabled ultrasound probe system developed by Mobisante as a Class II device. The mobile ultrasound imaging system will cost between $7,000 and $8,000 in full, which includes a Toshiba Windows Mobile-powered smartphone, an ultrasound probe and the company’s software. Read More
June 23, 2011: Zephyr inks a deal with AT&T to add cellular connectivity to its remote monitoring offering. Read More
July 14, 2011: Proteus Biomedical announces that it received a patent for its ingestible biomedical sensor — what the company has referred to as “intelligent medicine” in the past and what many have referred to as a “chip in a pill.” The sensor communicates through the person's body when it is swallowed and a patch on the patient's skin communicates the data to a companion smartphone app. Read More
 September 26, 2011: Sleep monitoring and tracking company Zeo announces a new product, called Zeo Mobile, that enables users to capture data from their Zeo headband right to their smartphones -- no bedside display alarm clock needed. Read More
September 26, 2011: Sleep monitoring and tracking company Zeo announces a new product, called Zeo Mobile, that enables users to capture data from their Zeo headband right to their smartphones -- no bedside display alarm clock needed. Read More
October 3, 2011: Fitbit launches its second generation activity tracker, Ultra, and its first iPhone app, which the wearable device does not connect to directly. Instead, the data is sent to Fitbit's online portal first via a wireless-enabled USB hub on the user's computer and the data is then updated for viewing via the app. Read More
October 10, 2011: Mobisante commercially launches its smartphone-enabled ultrasound system about eight months after securing FDA clearance. Read More
November 3, 2011: Jawbone commercially launches its first iteration of UP. Read More
November 9, 2011: Scanadu, a San Francisco-based startup that aims to develop a handheld smartphone-connected diagnostic device similar to the Star Trek tricorder, raises $2 million from a number of angel investors, including Playfish co-founder Sebastien De Halleux. Read More
November 23, 2011: Mountain View, California-based Glooko announces plans for a cord that will connect various off the shelf glucometers with the iPhone. The team includes a former Lifescan executive who led the J&J company's own iPhone-enabled glucose meter app initiative. Read More
December 7, 2011: The FDA clears AgaMatrix’s iPhone-enabled glucose meter iBGStar, which is co-developed with French pharmaceutical company Sanofi. The companies began offering the device in Europe earlier this year. In Europe it is available in Germany, France, Switzerland, the Netherlands and Italy. Read More
Tools for patient generated health data in 2012
 January 5, 2012: BodyMedia announces plans to offer a disposable, peel-and-stick, biometric patch developed in conjunction with medical device company Avery Dennison. The companies expect the device to be used in preliminary evaluations for weight management. Now known as the VUE patch, that product is still yet to be released commercially. Read More
January 5, 2012: BodyMedia announces plans to offer a disposable, peel-and-stick, biometric patch developed in conjunction with medical device company Avery Dennison. The companies expect the device to be used in preliminary evaluations for weight management. Now known as the VUE patch, that product is still yet to be released commercially. Read More
January 13, 2012: UK-based retail pharmacy chain Lloyds Pharmacy inks a deal with Proteus Biomedical to launch Proteus’ first commercial product, Helius, an offering that includes sensor-enabled pills, a peel-and-stick sensor patch worn on the body, and a mobile health app. The patch records when a pill is ingested, tracks sleep patterns, and records physical activity levels. Read More
January 30, 2012: Glooko, which offers a simple glucose monitoring logbook app and a cable that connects meters to iPhones, raises $3.5 million in its first round of funding, led by The Social+Capital Partnership, and including return backers Bill Campbell, Vint Cerf, Judy Estrin and Andy Hertzfeld, Venky Harinarayan, Russell Hirsch and Xtreme Labs. The Social+Capital Partnership’s founder and managing partner, Chamath Palihapitiya, has joins Glooko’s board of directors, too. Read More
January 31, 2012: An iPhone app from McLean, Va.-based vendor Vignet, that controls wireless home health monitoring devices, becomes the first Apple iOS product to meet Continua Health Alliance interoperability standards for connecting to a wide range of medical devices. It is also the first software product of any kind to incorporate Continua guidelines for interoperability of mobile applications over wide-area networks. Read More
April 3, 2012: Germany-based Medisana announces the planned launch of ThermoDock, an iPhone-connected non-contact infrared thermometer, making it available for pre-order in the UK for about US $96. At the time, the device does not have FDA clearance. Read More
April 20, 2012: BodyMedia and Withings partner up to integrate weight data from Withings’ WiFi scale into BodyMedia users' online dashboards and apps. BodyMedia offers its own suite of connected fitness devices, but has not developed its own connected weight scale. At this time, Withings offers a WiFi scale, a connected blood pressure monitor, and a baby monitor device, but no fitness tracker. Read More
April 23, 2012: Connected fitness device maker Fitbit announces the commercial launch of its Aria WiFi Smart Scale. Like competitor Withings, Fitbit Aria recognizes up to eight different user profiles and automatically determines which user is on the scale based on previous usage. Read More
May 23, 2012: Wearable body monitor company BodyMedia raises an additional $9.3 million to complete a $12 million funding round. Newcomer Comcast Ventures leads the round, which also includes previous investors Draper Fisher Jurvetson ePlanet, Draper Triangle Ventures, Ascension Health Ventures and InCube Ventures. Read More
 May 30, 2012: Glooko, a mobile diabetes management startup, inks a deal with Roche Diabetes Care to extend the Glooko MeterSync to Accu-Chek meter users via an infrared data transfer adapter. The Glooko IR Adapter helps iPhone or iPod touch users who would like to more easily download blood glucose readings from their Accu-Chek meters to the Glooko Logbook app on their iOS device. Read More
May 30, 2012: Glooko, a mobile diabetes management startup, inks a deal with Roche Diabetes Care to extend the Glooko MeterSync to Accu-Chek meter users via an infrared data transfer adapter. The Glooko IR Adapter helps iPhone or iPod touch users who would like to more easily download blood glucose readings from their Accu-Chek meters to the Glooko Logbook app on their iOS device. Read More
June 11, 2012: Khosla Ventures invests $1 million in CellScope, an alum from Rock Health’s first class of startups in 2011. CellScope was developing smartphone peripheral devices designed for consumers to use for at-home diagnosis, starting with a smartphone-enabled otoscope that would enable physicians to remotely diagnose ear infections in children. Read More
June 27, 2012: Glooko officially launches its MeterSync Cable and its companion Logbook app in Europe, which means iPhone users in European countries can now connect a handful of some of the most popular glucose meters to their phones to automatically upload data to a logbook app on the devices. Read More
July 2, 2012: Asthmapolis receives FDA 510(k) clearance for its asthma sensor and companion software. The company’s device is a sensor that sits atop (most) inhalers used by patients who have asthma or COPD. The sensor transmits data to a companion app on the user’s mobile phone every time the inhaler is used. The app can then track the time and location of each medication discharge, which can then be used to help patients and their caregivers better understand their asthma triggers. Read More
July 9, 2012: iHealth Lab, a subsidiary of China-based Andon Health, receives 510(k) clearance from the FDA for a wrist-worn, Bluetooth-enabled blood pressure monitor called the iHealth BP7 Wireless Blood Pressure Wrist Monitor that connects with iPhones, iPads, and iPod touch devices. Read More
 July 27, 2012: Cambridge, Massachusetts-based EyeNetra, which is developing what it calls the most affordable mobile eye diagnostic tool ever developed, raises $1 million of a hoped for $1.2 million round of funding from two undisclosed investors. The company’s peripheral device and software enables anyone to take their own eye test, get a prescription for glasses, and connect to eye-care providers right from their mobile phone. Read More
July 27, 2012: Cambridge, Massachusetts-based EyeNetra, which is developing what it calls the most affordable mobile eye diagnostic tool ever developed, raises $1 million of a hoped for $1.2 million round of funding from two undisclosed investors. The company’s peripheral device and software enables anyone to take their own eye test, get a prescription for glasses, and connect to eye-care providers right from their mobile phone. Read More
July 30, 2012: Proteus Digital Health, formerly known as Proteus Biomedical, becomes the first company to receive FDA clearance for an ingestible biomedical sensor that monitors medication adherence. The FDA grants 510(k) premarket approval to the Proteus Ingestible Event Marker (IEM) as a de novo medical device, meaning that there is no similar product on the market, four years after Redwood City, Calif.-based Proteus first sought clearance. Read More
September 27, 2012: Glooko announces an updated version of the Glooko Logbook app and support for an additional six blood glucose meters. The Glooko meter can now connect 17 different meters to iOS devices with its $40 Glooko MeterSync Cable. The newly supported meters include two new meters from Walmart: the ReliOn Confirm and ReliOn Prime. Read More
October 25, 2012: Massachusetts General Hospital in Boston conducts a clinical trial of wearable health monitoring devices from Zephyr Technology to monitor pregnant women as they give birth, with plans to quickly roll the system out to a hospital in Uganda in an effort to stem high infant mortality. Zephyr's BioHarness and ZephryLife monitoring system measure movement, respiratory rate, heart rhythm and other vital signs to help health workers identify conditions such as arrhythmia, pressure ulcers and potential falls early. Read More
November 13, 2012: A Beijing-based medical device company called Raiing is granted 510(k) FDA clearance for the Raiing Wireless Thermometer, a peel-and-stick contact thermometer sensor that continuously transmits body temperature readings to a companion iPhone app. Read More
November 29, 2012: Scanadu unveils prototypes of its first three products: The Scanadu Scout, its core tricorder offering, the ScanaFlo urine analyzer and the ScanaFlu influenza test. Read More
December 3, 2012: The FDA grants a 510(k) Class II clearance to San Francisco-based AliveCor’s iPhone-enabled heart monitor, which has been commonly known as the “iPhoneECG” since it first made an appearance at CES in 2010. The company only secures clearance for prescription use, and makes the device available for doctors to preorder. Read More
December 13, 2012: Masimo, a medical device maker founded in 1989, releases a commercially-available iOS-enabled pulse oximeter called the iSpO2. The company opts not to go for FDA clearance, saying the device is intended for use by climbers and pilots, not in medical situations. Read More
Tools for patient generated health data in 2013
 January 7, 2013: At the Consumer Electronics Show (CES) in Las Vegas, BodyMedia announces the Core 2 Armband, a smaller, more streamlined version of the company’s wearable tracker. Read More
January 7, 2013: At the Consumer Electronics Show (CES) in Las Vegas, BodyMedia announces the Core 2 Armband, a smaller, more streamlined version of the company’s wearable tracker. Read More
January 7, 2013: iHealth Labs, a California-based subsidiary of China’s Andon Health announces it is expanding its line of iOS-enabled wireless health peripherals. The company adds a wireless blood glucometer and a wireless pulse oximeter to their product offerings, though FDA clearance is still pending for both devices. Read More
January 7, 2013: Health devices are everywhere at CES in Las Vegas, including PerformTek's iriver, a fitness sensor-laden Bluetooth headset; Spree, an activity tracking headband; the Fitbug Orb; the Fitlinxx Pebble; Zensorium's Tinke pulse oximeter; Basis Band; GeoPalz family activity tracker and companion app; and Interaxon Muse, a smartphone-connected brainwave scanner. Read More
January 8, 2013: Beam Technologies, of Louisville, Ky., begins selling its manual brush with embedded brushing sensor and Bluetooth radio, for $49.99. A free companion app for Android or Apple iOS tracks brushing habits, including frequency and duration, as well as time spent in each quadrant of the mouth. Users sync data to the smartphone app by pushing a button on the brush handle. Read More
January 22, 2013: The iExaminer System from Welch Allyn, an iPhone app and peripheral device that allows doctors to use the iPhone camera to take photographs of the interior surface of the eye receives 510(k) FDA clearance. It builds on the company’s existing PanOptic Opthalmoscope, a device that lets a physician see into the back of a patient’s eye. Read More
February 14, 2013: A new ECG device for the iPhone 4s called ECG Check receives over-the-counter clearance from the FDA. The device, which would be the first product from Park City, Utah-based Cardiac Designs, appears very similar to the AliveCor Heart Monitor, which is currently only cleared for prescription use. Read More
March 4, 2013: Johnson & Johnson company LifeScan announces the launch of a Bluetooth-enabled glucose meter and companion iPhone app similar to the one the company showed off on-stage at an Apple World Wide Developer’s Conference in 2009 for the launch of iPhone 3.0. Read More
March 12, 2013: A WellPoint health plan in Florida, called Amerigroup Florida, announces that it will offer Asthmapolis’ FDA-cleared mobile health device and service to its members with asthma. Asthmapolis’ device is a sensor that sits atop (most) inhalers used by patients who have asthma or COPD. The sensor transmits data to a companion app on the user’s mobile phone every time the inhaler is used. Read More
March 12, 2013: MobiHealthNews breaks the news that sleep company Zeo shuts down. The Better Business Bureau lists Zeo as being “out of business” and Zeo CEO Dave Dickinson participates in an online TEDMED event as the company’s “former CEO”. It’s clear that Zeo as we knew it is over. Read More
 March 27, 2013: Scripps Health cardiologist Dr. Eric Topol shows off some smartphone-connected health devices, including CellScope and AliveCor, on Comedy Central's "The Colbert Report." It's the latest in a slew of mainstream media features on digital health, including Rock Center with Brian Williams and CNN's "The Next List." Read More
March 27, 2013: Scripps Health cardiologist Dr. Eric Topol shows off some smartphone-connected health devices, including CellScope and AliveCor, on Comedy Central's "The Colbert Report." It's the latest in a slew of mainstream media features on digital health, including Rock Center with Brian Williams and CNN's "The Next List." Read More
April 10, 2013: Dublin, Ireland-based Zinc Software announces a seed round of about $850,000 (€650,000) in funding from Kernel Capital, AIB Seed Capital Fund, and Enterprise Ireland. Zinc is developing wearable health sensors, including the Zen Sensor, which clips to a user’s ear lobe to collect heart rate data. Zinc also announces that it entered product development and supply chain management company PCH International’s accelerator program. Read More
April 11, 2013: Redmond, Washington-based Mobisante announces the launch of a tablet-enabled version of its portable ultrasound system, which received FDA 510(k) clearance in early 2011. The tablet version of the offering enables physicians to use more powerful ultrasound probes and, of course, brings a larger screen for review of high-resolution ultrasound imaging. Read More
April 17, 2013: Kinsa Health, a thermometer that connects to a smartphone via the headphone jack launches on Indiegogo. By using the phone’s battery and electronics, the company claims it has been able to build an accurate digital thermometer with a low price point — possibly even cheaper than non-connected digital thermometers are today. Read More
April 30, 2013: San Francisco-based Jawbone acquires Pittsburgh-based BodyMedia, for an undisclosed sum. This is the first major consolidation in the contentious wearable activity tracker space, and the second mobile health acquisition by Jawbone, which bought health app startup Massive Health for an undisclosed sum in February. Read More
May 8, 2013: At the American Telemedicine Association in Austin, Texas, Nonin shows off its newest product, the Nonin 3230 Bluetooth Smart-enabled pulse oximeter. The product is still awaiting FDA 510(K) clearance, but the product is on track to be the first wireless pulse oximeter to use the low energy communication protocol, also known as Bluetooth 4 or Bluetooth LE. Read More
May 10, 2013: Israel-based LabStyle Innovations, which is developing a smartphone-based glucose meter, raises $10 million by common stock that the company plans to accredited investors. The company plans to use the money to develop, market and manufacture Dario, its smartphone-enabled diabetes management system. Dario does not have a CE mark nor does it have FDA approval. Read More
 May 22, 2013: Scanadu launches an Indiegogo campaign to sell pre-orders of their tricorder device, the Scanadu Scout. In an unprecedented move, the company launches its crowdfunding campaign without FDA 510(k) clearance, with the intention of conducting the usability tests needed to get the clearance via the campaign. It makes its funding goal within two hours. Read More
May 22, 2013: Scanadu launches an Indiegogo campaign to sell pre-orders of their tricorder device, the Scanadu Scout. In an unprecedented move, the company launches its crowdfunding campaign without FDA 510(k) clearance, with the intention of conducting the usability tests needed to get the clearance via the campaign. It makes its funding goal within two hours. Read More
May 23, 2013: Researchers at the University of Illinois at Urbana-Champaign develop a versatile iPhone-based biosensor that, with about $200 worth of parts, is just as accurate as a $50,000 laboratory spectrophotometer. The system, consisting of an iPhone cradle and an app, can detect viruses, bacteria, toxins, proteins and even allergens in food using the smartphone’s camera as a spectrometer and the powerful processor to make calculations. Read More
May 24, 2013: Two devices had crowdfunding campaigns going at this time, including an ultrasound doppler that plugs into the iPhone’s headphone jack, so pregnant women can hear their baby’s heartbeat at home and an app that helps people with diabetes monitor medication habits and intelligently autopopulates fields to minimize the amount of input required. Read more
May 30, 2013: Polar, the longtime makers of heartrate-monitoring watches and chest straps, launched a Bluetooth Smart-enabled, iPhone-connected activity tracker. The Polar Stride Sensor Bluetooth Smart is a small device that clips to the shoe and tracks stride-length, speed, running cadence, and distance. Read More
July 9, 2013: New York-based Pixie Scientific developed urine-tracking Smart Diapers. The diapers have a code on the bottom that will change colors after the baby pees. From there, parents need to scan the diaper with a companion app to log the information. While Smart Diapers tests for urinary tract infection, prolonged dehydration and developing kidney problems, it will also look for health patterns that will be visible after a few months of tracking. The company has since developed Pixie Briefs for adults. Read More
August 14, 2103: Lumo Body Tech, makers of an app-connected posture sensing belt, launched a new, overhauled version of its LUMOback sensor today. The new sensor is a significant hardware upgrade and also includes new tracking capabilities and app features. The new version has replaced the original LUMOback, which is no longer being sold online. It still retails for $149.95 and is compatible with iOS devices from the iPhone 4S onwards. Read More
September 12, 2013: Two crowdfunding campaigns are underway at this time, including a Bluetooth headset that lets a user listen to music and make phone calls, but also tracks health metrics including breath patterns, heart rate, and pulse oximetry and a machine washable shirt that measures activity, heart rate, ECG, and breathing. Read More
October 16, 2013: After reducing its FDA 510(k)-cleared smart toothbrush from $49.99 down to $24.99, Beam Technologies released its new product for pre-orders at a $19.99 price point. The Beam Brush Bug, which the company informed customers about in an email, is a small adhesive accelerometer they can attach to any toothbrush or flossing device. Read More
October 29, 2013: Diabetes tracking company Glooko, which offers a cloud-based logbook app and a cable that connects the iPhone to commercially available blood glucose monitors, launched, and received FDA 510(k) clearance for, a new app and cable for Android phones. Glooko’s new app connects to the same cloud-based web dashboard as the company’s iPhone app. That platform was FDA cleared in May and launched in June. Read More
October 30, 2013: iHealth Labs finally releases it’s Bluetooth-enabled, smartphone-connected glucometer first announced nearly a year previously at CES in Las Vegas. The device, called the Wireless Smart Gluco-Monitoring System, received FDA 510(k) clearance in April. The device is available on iHealth’s website and also in Best Buy stores. The company said it will be available at a “leading national drug retail chain.” It costs $79.95, which includes 50 test strips and 25 lancets. Read More
 November 13, 2013: Orpyx Medical Technologies, of Calgary, Alberta, introduced the SurroSense Rx system to U.S. customers only through its website, in men’s sizes 5-13 and women’s sizes 6-12. SurroSense Rx collects data about where people are exerting pressure on their feet—too much heel pressure can cause foot numbness—and alerts wearers when they are at risk for diabetic foot ulcers that could lead to amputation. Orpyx is currently working with a number of clinics and hospitals across the US on a controlled beta launch. Read More
November 13, 2013: Orpyx Medical Technologies, of Calgary, Alberta, introduced the SurroSense Rx system to U.S. customers only through its website, in men’s sizes 5-13 and women’s sizes 6-12. SurroSense Rx collects data about where people are exerting pressure on their feet—too much heel pressure can cause foot numbness—and alerts wearers when they are at risk for diabetic foot ulcers that could lead to amputation. Orpyx is currently working with a number of clinics and hospitals across the US on a controlled beta launch. Read More
November 20, 2013: Medical device maker Nonin announced a new smartphone-connected product, a regional oximeter, at Medica in Dusseldorf, Germany. Regional oximetry, also called cerebral oximetry or rSO2, is a form of continuous monitoring that measures the level of oxygen saturation in the brain or on other tissue. Read More
November 27, 2013: The University of California at Los Angeles Wireless Health Institute teams up with smart cane startup Isowalk to create a sensor-laden cane that could be used to predict falls or help speed up recovery for injured athletes. The Isowalk is currently registered as a Class I device with the FDA. Some applications, particularly clinical ones, may require the device to also secure 510(k) clearance as a Class II device, and UCLA is currently working on those applications. They foresee a third quarter 2014 release for the device. Read More
December 5, 2013: The FDA clears the eMotion ECG Mobile, a Finnish continuous ECG monitor that connects to Android smartphones, as a Class 2 medical device. The ECG Mobile, made by Mega Electronics, already has a CE Mark and has been available for purchase in Europe since late 2012, but the company’s securing of FDA clearance suggests it may be bringing the device to the United States to compete with mobile ECG platforms like the well-established AliveCor or as-yet-unlaunched Cardiac Designs. Read More
December 16, 2013: San Francisco-based IntelliClinic crowdfunds a brainwave-sensing smartphone-connected sleep mask. The device, called NeuroOn, raises $338,573 over its $100,000 goal. It’s a foam sleep-mask that contains several different sensors and a Bluetooth transmitter. It offers users several different ways to use the data it collects. The mask contains an electroencephalographic (EEG), or brain wave, sensor as well as sensors that measure the movement of the users’ eyes and facial muscles. Read More
December 18, 2013: Fertility app maker Glow, which first launched in August, announces that its app has helped more than 1,000 women conceive. The reported pregnancies have come from across the United States and other countries including Canada, the United Kingdom, and Tanzania. Glow accompanied the announcement with the launch of a new version of the app, which was redesigned for iOS7 and includes a more accurate fertility window prediction in the calendar. Read More
December 18, 2013: Helsinki, Finland-based sleep sensor company Beddit is on track to fulfill its promise to ship its product to its Indiegogo backers by the end of the year, the company says, and is opening the door for additional pre-orders via Amazon, its own online store and a number of resellers. The company, which makes a sensor that straps to the user’s bed, raised $503,000 this year in an Indiegogo campaign with an original goal of $80,000. Now it’s opening two online stores, one for the United States and one for Europe, and accepting Amazon pre-orders. Whereas Indiegogo backers paid a discounted $99 for the sensors, preorders are listed at the full $149. They’re expected to ship in January 2014. Read More
Tools for patient generated health data in 2014
 January 2, 2014: New York City-based Kinsa Health receives FDA 510(k) clearance for its Kinsa smart thermometer. Kinsa’s device is not the first smartphone-connected thermometer to get FDA clearance: MobiHealthNews reported on the Raiing Wireless Thermometer’s 510(k) clearance back in November 2012. Kinsa might well be the first smartphone-connected oral thermometer to receive clearance, however, as Raiing’s device is a continuously-monitoring skin patch. Kinsa’s device can be used, orally, under-arm, or rectally. The thermometer also connects to a smartphone via a headphone jack, and uses the smartphone’s electronics, allowing the device itself to be sold very cheaply. Read More
January 2, 2014: New York City-based Kinsa Health receives FDA 510(k) clearance for its Kinsa smart thermometer. Kinsa’s device is not the first smartphone-connected thermometer to get FDA clearance: MobiHealthNews reported on the Raiing Wireless Thermometer’s 510(k) clearance back in November 2012. Kinsa might well be the first smartphone-connected oral thermometer to receive clearance, however, as Raiing’s device is a continuously-monitoring skin patch. Kinsa’s device can be used, orally, under-arm, or rectally. The thermometer also connects to a smartphone via a headphone jack, and uses the smartphone’s electronics, allowing the device itself to be sold very cheaply. Read More
January 3, 2014: Basis Science announces a new advanced sleep analysis feature for its wristworn, multisensor health tracker as well as a new Carbon Steel Edition of the device that it promises will fit more snuggly and look more premium than its more plastic predecessor. Read More
January 6, 2014: Redmond, Washington-based Heapsylon partners with shoe company Vivobarefoot to sell Heapsylon’s Sensoria Fitness sensor-enabled “smart” socks and to further develop the product. The company says that all of the stores worldwide that currently carry Vivo’s product will soon have Sensoria products in them, too. According to Vivo’s website, there are nearly 500 such stores around the world. The smart socks track a user’s steps, speed, calories, altitude and distance while running or walking through a combination of a sensor-laden sock, electronic anklet piece and an app available on iOS and Android platforms. On the companion app, which is connected to the sock via Bluetooth Smart, a user can see his or her cadence, foot landing technique and weight distribution, which the company hopes will help users identify if they have an injury-prone running style. Read More
January 7, 2014: French connected health and wellness device company Withings announces two new products planned to launch in 2014: a Bluetooth-enabled update of the company’s upper arm blood pressure cuff and a two part sleep tracking system. The sleep system, called Withings Aura consists of a sensor placed in the user’s bed and a bedside device that serves as both lamp and alarm clock. The system is controlled via a smartphone app. The blood pressure cuff is similar to the one Withings released in 2011, but adds Bluetooth connectivity and Android support (the previous device connected via the phone’s 16-pin connector). The cuff has a CE Mark and is waiting on FDA clearance. Read More
January 8, 2014: Once again, many different connected health devices launch at CES this year. iHealth Lab shows off three new smartphone-enabled, wearable health devices: a blood pressure monitoring vest, an ambulatory ECG device that (assumedly) sticks to the wearer’s bare chest, and a wristworn pulse oximeter device. Posture startup Lumo Body Tech previews its new tracker Lumo Lift. This device is considerably smaller than its predecessor, and instead of taking the form factor of a belt, the new device uses a magnetic clasp to clip to the wearer’s clothing. Zensorium announces its consumer pulse oximeter for Android, Tinke. And Wellograph shows off its “wellness watch” -- a health-tracking smartwatch with an LED screen that displays an analog or digital clockface centrally and fitness statistics on the bottom of the screen. In addition to an accelerometer, it includes a continuous heart rate sensor. Read More
January 14, 2104: Mattress maker Sleep Number announces a smart bed, called x12, in partnership with sleep tracker maker Bam Labs. Prices for the bed will start at $7,999.99. Bam Labs’ sleep tracker product has formerly only been used in hospital bed systems. Sleep Number’s mattress uses technology called SleepIQ to track and monitor each person’s sleep, show what kinds of comfort adjustments each person can make, and suggest changes the user can make to their daily routine. The bed also tracks each person’s average breathing rate, movement and average heart rate. Read More
January 17, 2014: Google officially announces its intentions to develop smart contact lenses, as a noninvasive method of measuring blood glucose levels in people with diabetes. The contact lens is comprised of a miniturized glucose sensor and wireless chip embedded between two layers of contact lens material, although the cofounders add that eventually it might have tiny LED lights that would act as an early warning when glucose levels have dropped or risen past certain thresholds. The announcment is met with skepticism from the continuous glucose monitoring research community. Read More
January 17, 2014: Four devices had crowdfunding campaigns going at this time, including a sensor that can analyze a user’s vitals through touch, and also identify diseases and environmental factors such as pollution; a blood pressure and heart rate monitor that aims to not only be accurate, but also beautiful, so that it fits into the user’s daily life; smart cable and app that help people with diabetes better manage their health and condition; a weight scale, measures eight body statistics — weight, body mass index, fat-lean ratio, water ratio, bone ratio, calorie, muscle ratio, and visceral fat ratio; and a spirometer that connects to a smart phone, provides a two-step solution for monitoring respiratory problems. Read More
 January 27, 2014: Palo Alto, California-based SleepRate launches a sleep app, based on sleep analysis algorithms and Cognitive Behavioral Therapy for Insomnia (CBTI) protocols, which were licensed from Stanford University’s School of Medicine, Psychiatry, and Behavioral Sciences. The system is available for $99.99 in SleepRate’s online store or on Amazon.com, although only to those in the United States. SleepRate’s system includes a Polar H7 Bluetooth heart rate monitor and a smart alarm, which aims to help wake people up at the right time in their sleep cycle. Read More
January 27, 2014: Palo Alto, California-based SleepRate launches a sleep app, based on sleep analysis algorithms and Cognitive Behavioral Therapy for Insomnia (CBTI) protocols, which were licensed from Stanford University’s School of Medicine, Psychiatry, and Behavioral Sciences. The system is available for $99.99 in SleepRate’s online store or on Amazon.com, although only to those in the United States. SleepRate’s system includes a Polar H7 Bluetooth heart rate monitor and a smart alarm, which aims to help wake people up at the right time in their sleep cycle. Read More
February 10, 2014: Researchers Takayasu Sakurai and Takao Someya develop a flexible, disposable sensor for diapers. The sensor, which can detect moisture, humidity and pressure, is printed on plastic film and will transmit information wirelessly. At some point, the developers expect that their devices could be created through inkjet printing, which would lead to a reduction in manufacturing cost. Next steps for the researchers include improving the reliability of the battery and reducing the power consumption of the sensors. Read More
February 21, 2014: Dental hygiene company Oral-B announces its Bluetooth-connected electric toothbrush line, SmartSeries, which will be available in Germany this spring and in the United States in June. The Oral-B companion app will be available in iOS in May, and in Android in August. Oral-B will also add connectivity to some of its other electric toothbrushes. Read More
February 24, 2104: Samsung announces two smartwatches, Samsung Gear 2 and Gear 2 Neo, which launched in April. The main differences between the two devices is Samsung Gear 2 comes equipped with a camera and the Gear 2 Neo does not. The company also makes some general changes to its original smartwatch Galaxy Gear, such as switching operating systems, from Google’s Android to Tizen and extending the phone’s battery life. But Samsung also adds another health-related feature — a heart rate monitor. Like the original smartwatch, Samsung’s latest offerings also come equipped with pedometer features. Read More
February 25 2104: Verizon receives its second FDA clearance for its converged health management software platform. While the intended use cases and overall technical aspects of the system haven’t changed, Verizon has added support for Telcare’s cellular-enabled blood glucose monitoring system and Genesis Health Technologies’ blood glucose monitoring system. The device’s first clearance enabled it to connect to five devices from Ideal Life — its blood pressure cuff, glucose monitor, pulse oximeter, weight scale, and its telehealth hub. The recent additions point to Verizon’s goal of having a device-agnostic platform. Read More
February 25, 2014: Samsung adds a heart rate sensor to its Galaxy S5 smartphone. This new iteration has many health features on top of its built-in heart rate sensor, such as the next version of Samsung’s S Health app and a pedometer. Read More
March 5, 2014: Three devices had crowdfunding campaigns going at this time, including one that allows patients to track vital signs, take notes about treatments, store medical records, and book appointments; a wearable thermometer for women tracking their basal body temperature; and a device and app that helps people determine the level of pesticides and insecticides in food, whether drinking water contains harmful chemicals, and the quality of air. Read More
March 6, 2014: Jawbone, maker of the Jawbone UP activity tracker launches an app called UP Coffee. The app helps users regulate their caffeine intake and understand how caffeine affects their sleep. While the app is open for anyone to download, not just owners of a Jawbone UP wristband, if the user owns the UP or UP24, he or she can also sync UP Coffee with that device. UP Coffee provides users with more information about how their caffeine intake affects sleep, the more they log in the app. Read More
March 10, 2014: San Francisco-based Azoi announces Wello, a smartphone case that measures a user’s vitals including blood pressure, electrocardiogram (ECG), heart rate, blood oxygen, and temperature. The device is waiting for FDA clearance, but in the meantime users can preorder it for $199. The company plans to ship devices to the US after they receive FDA clearance, which they expect in fall 2014. Azoi expects to ship the device to the UK, Singapore, Canada, Hong Kong, China, and India starting in the summer of 2014. Read More
 March 20, 2014: Remote patient monitoring company HealthInterlink receives FDA 510(k) class II clearance for Beacon 2.0, a mobile-centric software system that integrates data from various home health devices. Beacon was previously cleared as a class I medical device (MDDS). HealthInterlink plans to begin commercialization of the device in the US immediately. Read More
March 20, 2014: Remote patient monitoring company HealthInterlink receives FDA 510(k) class II clearance for Beacon 2.0, a mobile-centric software system that integrates data from various home health devices. Beacon was previously cleared as a class I medical device (MDDS). HealthInterlink plans to begin commercialization of the device in the US immediately. Read More
March 25, 2014: Intel confirms its rumored acquisition of Basis Science, the activity tracker company that makes the high-end Basis B1 Band. Intel Capital became an investor in Basis last October and joined the company’s board at that time. Other Basis investors included Norwest Venture Capital Partners, DCM, and Mayfield Fund. The startup raised just over $30 million in total. Read More
April 1, 2014: Withings secures FDA 510(k) clearance for its previously announced Wireless Blood Pressure Monitor and it has launched the device in the United States. As announced at CES 2014 in January, the blood pressure cuff is similar to the one Withings released in 2011, but adds Bluetooth connectivity and Android support (the previous device was iOS-only and had to be plugged into the Apple device). The device will sell for $129.95. Read More
April 2, 2014: Three devices had crowdfunding campaigns going at this time, including a wearable brain training system that fits into an existing hat or headband and contains three EEG sensors, a handheld, smartphone connected blood spectrometer, and a wearable thermometer for children. Read More
April 15, 2104: Beijing-based Giraffe Tech raises $16,000 on a crowdfunding platform, called Demohour for a posture-sensing wearable device, called Giraffe Friend. Giraffe Friend was developed by Xiang Renkai, a student at Peking University, who was inspired by a Chrome plug-in, which allows users to browse the internet using motion recognition technology. The company expected to ship the product a month after the campaign ended. Read More
April 17, 2014: Biopharmaceutical company Opko Health acquires Israeli smart inhaler company Inspiro Medical for a sum in “the low eight figures”, according to Opko’s Director of Strategic Investment Les Funtleyder. The company will be using Inspiro’s Inspiromatic technology to develop an app-connected inhaler that will be bundled with a forthcoming new drug for asthma, COPD, and cystic fibrosis. Read More
April 23, 2104: Withings launches the next version of its Pulse activity tracker, now called Pulse O2. The new device allows users to measure their blood oxygen levels, which help users understand the efficiency of their overall lung functions. This tool is especially useful for people who work out in high altitudes, for example mountain climbers, or people with respiratory issues, such as asthma or chronic bronchitis. Withings said the device can guard against hypoxia, a condition in which the body is deprived of oxygen, but Withings has not had Pulse O2 cleared with the FDA. Pulse O2 is on sale over at Withings’ website for $119.95. Read More
April 30, 2104: Three former Dexcom employees leave the company last year to form a new venture, Glucovation, which is developing a direct-to-consumer, wearable device that continuously senses glucose for people trying to lose weight or improve their athletic performance. The Glucovation team is headed up by Dexcom’s former senior technical director for R&D, Robert Boock, who was in charge of that company’s future generation sensor products. The company aims to keep its device’s pricepoint around $150 and the replaceable sensors at about $20 each. Read More
April 30, 2014: London-based digital health tool maker Big Health raises $3.3 million from Index Ventures and Forward Partners to expand into the United States. The company plans to open an office on the west coast. The company’s first tool, Sleepio, aims to help users fix their sleeping issues. Users are asked to take a sleeping test and then upload sleeping data from a fitness device, Jawbone UP, Fitbit, or BodyMedia tracker, if they have one. From there, Sleepio creates a personalized program for the user to follow. Read More
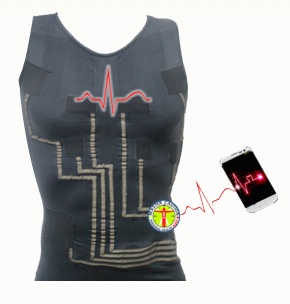 May 5, 210: Tel Aviv, Israel-based HealthWatch is working on another entry for the still emerging smart textile field. At the American Telemedicine Association conference in Baltimore, Maryland, the startup previews its hWear line of T-shirts with interwoven ECG sensors, allowing the shirt to function as a 3 to 15 lead ECG. The garment itself is registered with the FDA as a Class I device, but the company is seeking 510(k) clearance for the complete system. HealthWatch estimates the cost of the shirts will be around $200 to the end user. In the future, the company is also looking at designing a version for pregnant women that could be used for fetal ECG monitoring. Read More
May 5, 210: Tel Aviv, Israel-based HealthWatch is working on another entry for the still emerging smart textile field. At the American Telemedicine Association conference in Baltimore, Maryland, the startup previews its hWear line of T-shirts with interwoven ECG sensors, allowing the shirt to function as a 3 to 15 lead ECG. The garment itself is registered with the FDA as a Class I device, but the company is seeking 510(k) clearance for the complete system. HealthWatch estimates the cost of the shirts will be around $200 to the end user. In the future, the company is also looking at designing a version for pregnant women that could be used for fetal ECG monitoring. Read More
May 5, 2014: Medical device giant Covidien acquires sports and medical wearables company Zephyr Technology. The company has raised more than $13 million since its founding in 2003 — back when health-sensing wearables were a relative rarity. Zephyr’s investors included 3M New Ventures, Alsop Louie Partners and Motorola Solutions Venture Capital. Read More
May 13, 2014: San Diego-based Cue launches an at-home lab test. The product is now available for preorder from their website. Cue is a modular at-home lab test, which, using a sample of blood, saliva, or mucus can conduct at home versions of five lab tests (to start with): influenza, testosterone, vitamin D, inflammation, and fertility. The information is then sent via Bluetooth to the user’s iOS or Android smartphone. The device is about the size of a Rubik’s cube, and the cartridges are about the size of a box of matches. Read More
May 13, 2014: South Korean technology company LG, launches its Lifeband Touch fitness tracker and heart rate sensing earphones, announced at CES. The devices will be available exclusively from some Best Buy stores. The Lifeband Touch interfaces with the user’s phone to inform the user about incoming calls and text messages, and allows the user to control his or her music from the wearable as well. Additionally, the earphones that come with the device have embedded sensors that track heart rate, a feature that few other fitness trackers offer. The feature might be appealing to serious runners, who could use the earphones in place of a chest strap heart rate monitor. Read More
May 20, 2014: Two devices had crowdfunding campaigns going at this time, including a headset that lets a user connect any stethoscope to record sounds of his or her heart, lungs, and GI tract and a holistic tracker that monitors fitness by measuring distance, calories burned, and heart rate; safety by tracking location, potential risks for sleep apnea, and automatic emergency alerts; health by gathering data on skin temperature, blood oxygen saturation, electrocardiography, and stress levels; and lifestyle choices by offering a barcode scanner, gesture control, and a method for payment. Read More
May 22, 2014: The Epilepsy Foundation launches a device and connected app on Indiegogo, called SAMi, that helps caregivers track family members who have recurring seizures (RS). The SAMi camera is placed in the patient’s bedroom and a companion app is used by the caregiver. At night, SAMi records the patient’s movements, but only alerts the caregiver of sustained periods of movement. The Epilepsy Foundation plans to use the funds to update the camera, create manuals, installation instructions, and an instructional video. The camera is available for preorder for $399 and the estimated shipping date is February 2015. Read More
May 27, 2014: Mountain View, California-based pregnancy tracking company Bellabeat raises $4.5 million from SVAngel, CrunchFund, Universal Music Group and angel investors. With the Bellabeat device, pregnant women can listen to and record their babies’ heartbeat and track other aspects of their pregnancy from the companion app, including movement, kicks, and prenatal care. The device is available for $129 from the Bellabeat website and comes with a gel to use when listening to the heartbeat. Read More
May 27, 2014: Samsung gives customers a new way to use the Galaxy S5′s built-in optical heart rate sensor: to detect stress. In a new update users can not only check their stress level, but map it out on an hourly, daily, or monthly graph. Read More
May 29, 2014: Samsung announces two new digital health projects at an event called Voice of the Body in San Francisco. One project is Simband, an “investigational device” — not a product – that is stocked with a variety of health sensors and room for third party developers to add their own. Samsung also unveils Samsung Architecture Multimodal Interactions, or SAMI, which it described as a “data broker” that future devices based on the Simband and other third party health tracking devices could upload data to that could then be used by app developers to create new apps. Read More
June 2, 2014: After months of rumors, Apple unveils a health data aggregating platform for developers called HealthKit and a companion app for consumers called Health. The Health app will come preloaded on all new iOS devices and any iPhone or iPad user who updates their devices to iOS8. While the company specifically mentioned integrations with the Mayo Clinic, Nike+, and Epic Systems, it plans to aggregate data from a wide range of third party devices. It's not clear if Apple will exclusively pick winners or if it will open the platform up to all comers, but it is likely any device sold in Apple's physical stores will feed data into HealthKit. Read More
















