 Chrono Therapeutics, a Hayward, California-based company working on a new wearable for smoking cessation and drug delivery, has raised $32 million in its first round of funding. The round was led by Canaan Partners and 5 am Ventures. Additional contributors included Fountain Healthcare Partners, and two strategic investors: GE Ventures and the Mayo Clinic.
Chrono Therapeutics, a Hayward, California-based company working on a new wearable for smoking cessation and drug delivery, has raised $32 million in its first round of funding. The round was led by Canaan Partners and 5 am Ventures. Additional contributors included Fountain Healthcare Partners, and two strategic investors: GE Ventures and the Mayo Clinic.
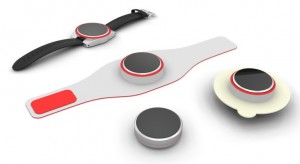
The company's flagship product SmartStop, which has been in development since 2004 and has been funded so far by a combination of NIH grants and the founders' personal income, is a wearable device that would smartly deliver nicotine to the wearer at strategic times.
The device can be worn on the wrist, arm, or as an adhesive patch. A smoker can wear the device and it would deliver progressively smaller doses of a nicotine over a 10-week period, much like existing nicotine replacement therapy patches. However, rather than releasing the drug continuously throughout the day, the patch will deliver doses at key times, like first thing in the morning and after meals, when smokers' cravings are most likely to occur. The objective is to not just alleviate cravings, but actually prevent them.
The wearable also connects to a smartphone and delivers information via Bluetooth about whether the user has been wearing the device and injecting the drug. That information, in turn, feeds into an app-based behavior change program designed to help smokers cope with cravings.
"We can send messages via [the user's] smartphone, text, voice, or email messages to remind them to take the product, if you haven’t used it in a day or two or you didn’t use it today and [up until now] you were doing so well," Chrono CEO Alan Levy told MobiHealthNews. "We can deliver encouragement, we can set up support networks of members’ friends and family, and that’s been shown very effective in getting people to quit. ... Dealing with the habit part of smoking, as well as the chemical dependence part of smoking. Our product deals with both aspects of smoking addiction. It’s the only product that does that."
Levy stressed that the platform could also be used for delivery of various other drugs for conditions including Parkinson's, asthma, or ADHD. In all cases, the company will work with drugs that are off patent and are therefore available relatively inexpensively. He said the funding will be used to get the product closer to launch, which is still a ways away.
"What we’ll do with the funds that we’re raising is first getting our hardware — the drug delivery product — to a form that it can be manufactured in a high volume," he said. "Also working with our internal people developing the back-end, the smartphone part of it, the compliance measurement and the behavioral support. Then we’ll be starting clinical trials and we expect to be able to submit and gain [FDA] approval within three years, with the money we’ve raised in this round."
They are not seeking FDA clearance as a medical device, but rather as a drug delivery method. The group will be working with the Mayo Clinic, an investor in the round, on their clinical trials. GE has made no commitments, but Levy says they are "very interested" in the device for their large self-insured employee population.
Levy says his company aims to charge between $400 and $500 for the therapy, about the same as other 10-week smoking cessation options.



















