 Boston-based Neumitra, which has developed a wearable that continuously measures the autonomic nervous system to track stress levels, has received an undisclosed amount of seed funding from the Thiel Foundation's Breakout Labs. Breakout Labs typically invests between $100,000 and $350,000 in its portfolio companies, which include Palo Alto, California-based "EKG for the gut" device maker G-Tech.
Boston-based Neumitra, which has developed a wearable that continuously measures the autonomic nervous system to track stress levels, has received an undisclosed amount of seed funding from the Thiel Foundation's Breakout Labs. Breakout Labs typically invests between $100,000 and $350,000 in its portfolio companies, which include Palo Alto, California-based "EKG for the gut" device maker G-Tech.
Neumitra's wearable, called neuma, currently measures skin conductance (galvanic skin response), ambient temperature, and tri-axis motion. Its battery currently lasts for about 50 continuous hours before it needs charging, which takes about four hours. In addition to its wristworn sensor, Neumitra makes use of mobile software to gather contextual data -- events, locations, and activities -- to map triggers of stress. The company says its mission is to develop a "validated stress score":
"We are all affected by too much stress, lost sleep, and follow on health concerns," the company writes. "Neumitra's mission is a validated stress score for daily brain health. We aim to address questions of daily brain health with objective data streams – from veterans with PTSD and mothers with newborns to CEOs with constant travel schedules and nurses with long work hours. Neumitra aims to address brain health concerns with data-driven technologies."
Neumitra's previous investors include the founders of Boston Scientific, Yahoo, and General Magic as well as digital health seed fund Rock Health. While the company's neuma device is currently available direct-to-consumer from its website at $1,500, Neumitra's primary target customers are researchers, Fortune 500 companies, and various healthcare organizations.
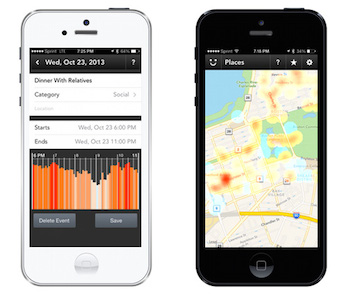
A few years ago at a MobiHealthNews event in Boston, Goldberg explained that Neumitra's wearable's companion app would also make suggestions to reduce stress, like listening to music or playing a game on the phone. He also said at the time that the company was working with public health departments to create stress maps of campuses and cities from the aggregate data generated by users.
Aside from a handful of high-level press interviews, Neumitra has remained relatively quiet since its founding in 2010. That may change soon as the years of development and early validation studies have positioned the company for commercialization.
"We believe biomodules that seamlessly blend into what's already being worn are much more likely to have ubiquitous applications than the typical branded products competing for the same bodily spaces. We saw when we started Neumitra in 2010 that continuous physiological monitoring had the potential to revolutionize questions of brain health and performance," CEO Robert Goldberg told MobiHealthNews in an email. "When we didn't find data quality meeting robust needs, we had to build our own hardware. Embedded biomodules are much more in line with that original vision. We found for instance only a small subset of users wanted to wear a digital watch, no matter how beautiful or smart we made it. Given the wide variety of health concerns made worse by acute and chronic stress, we knew we had to do much, much better."


















