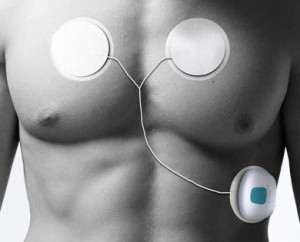
 Intelesens's Zensor device.
Intelesens's Zensor device.
Scanadu and Intelesens (makers of the Zensor device), two of the ten finalists for the Qualcomm Tricorder X Prize, have merged their teams into one as the competition enters the home stretch. It wraps up in 2016.
"Intelesens and Scanadu have been working together informally for a while," Shannon Wolf Montague, Commercial Manager at Intelesense, told MobiHealthNews. "At this point we believe our combined strengths give us a much better chance of winning than individually. Intelesens's background is in medical device R&D and regulatory approval, but the Tricorder X prize is [moving] more toward consumer products, where [Scanadu] have a lot of experience with their Scout and the user experience of their system."
Both Zensor, which purports to monitor ECG, respiration, and movement, and the Scout, which originally planned to measure pulse transit time, heart rate, electrical heart activity, body temperature, heart rate variability, and blood oxygenation, are still in the midst of their FDA clearance processes. While Zensor's 510(k) application is fairly traditional (Montague predicts clearance in three months), Scanadu's is somewhat novel: the company is in the process of shipping "investigational devices" to consumers in advance of FDA clearance.
Team Scanadu-Intelesens's final device will likely include the Scout and Zensor as a package, with the user interface app designed by Scanadu. The app will also incorporate algorithms from Intelesens that help streamline the diagnostic process.
Montague said the plan is for the companies to continue to work together after the competition when they bring their products to market, though she declined to comment on whether that would take the form of a partnership or a full-on merger.
"The direction of the partnership at this point is really taking medical technology to [the] consumer," she said. "We have a lot of experience in medical device commercialization. But the market is changing. The amount of adoption of medical technology by the consumer is increasing. For example, atrial fibrillation is an area we're looking at. At this point, people need to go to their doctor for AF screening. Now it’s starting to move toward 'if you can get the ECG at home and send that to a doctor via mobile, you’re in control of your own healthcare.'"
The Scanadu and Intelesens news isn't the only shake-up as the Tricorder X Prize nears its end. Slovenian team MESI Simplifying Diagnostics has dropped from the competition, in order to focus more fully on commercialization of its product, Tricorder X Prize Director Grant Campany told MobiHealthNews at the sidelines of HIMSS in Chicago this week. That brings the number of finalist teams down to eight: Scanadu-Intelesens, Aezon, CloudDX, Davantri, DMI, Dynamical Biomarkers Group, Final Frontier Medical Devices, and Scanurse.














