 We are holding Senate hearings to address reimbursement barriers for eCare and wireless health services, Dr. Mohit Kaushal, the director of healthcare at the FCC said during a presentation at the Wireless Life-Sciences Alliance here in La Jolla, CA.
We are holding Senate hearings to address reimbursement barriers for eCare and wireless health services, Dr. Mohit Kaushal, the director of healthcare at the FCC said during a presentation at the Wireless Life-Sciences Alliance here in La Jolla, CA.
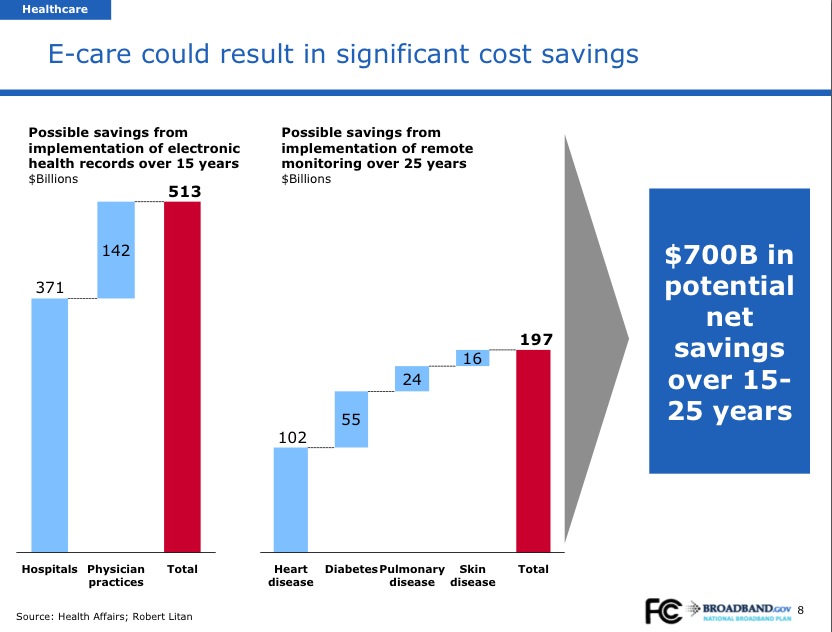
The federal government is going to focus on cost cutting, Kaushal said, as it might has allocated $10 billion through 2019 for the creation of a CMS Innovation Center. Kaushal said there will be more news on the Center come January of next year. Kaushal said that while CMS has conducted pilots in the past, it is very hard for pilots to get implemented at the "macro level," he said. The CMS Innovation Center will conduct pilots that reward value instead of volume, Kaushal said.
The CMS Innovation Center will be the most important piece for changing CMS reimbursement for wireless health services, Kaushal said.
Kaushal said another important barrier that needs to be resolved is the difficulty physicians have practicing medicine outside of their state. Kaushal said it's even difficult in some cases to practice outside of their specific hospitals. "We are working hard to remove those barriers," Kaushal said.
Another barrier on the regulatory front that Kaushal said his team was working on: The transparency in the process for approving devices. Kaushal said the murkiness of that process is prevent private investment interest in new health IT industries, including wireless health. Kaushal said that the FCC, FDA and industry groups plan to hold a workshop by the end of the summer that aims to fully define the unknowns when it comes to determining a "medical grade quality of service" or questions of privacy assurance.
The "culture" of the FCC's Broadband Plan is to protect consumer safety, Kaushal said, but it is also very pro-innovation. Kaushal said in some cases it will be very easy to tell where the FDA should step in to assess connected devices, including implantable wireless devices, while iPhone apps are not the type of thing that should be regulated by the FDA but by the FCC instead, Kaushal said.
"We have been wondering: Can we just streamline this whole process and maybe create a one-stop shop" for FCC and FDA approvals? Kaushal explained.
If it were truly streamlined, such a one-stop shop could be the regulatory clarity potential investors in mHealth have been waiting for.



















