 FDA and Health Canada recently cleared Calgary Scientific's Resolution MD app, the system is certified for clinical use by Health Canada and the FDA. The smartphone application is offered free of charge to doctors who are affiliated with hospitals and diagnostic labs that implement the solution.
FDA and Health Canada recently cleared Calgary Scientific's Resolution MD app, the system is certified for clinical use by Health Canada and the FDA. The smartphone application is offered free of charge to doctors who are affiliated with hospitals and diagnostic labs that implement the solution.
Update: The company is still seeking FDA approval for the app for handheld devices. A report over at Technology for Doctors is reporting that the FDA has cleared the app, however, the FDA said that is not accurate.
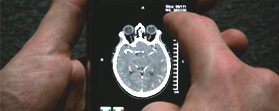
“Doctors tell us response times are five to 15 minutes before the PACS system locates and transmits the diagnostic image they want across the network to their workstations," Calgary Scientific CEO Byron Osing told Technology for Doctors in a recent interview. "If they’re using a smartphone in an acute care situation, pushing a 300 Mb image over a wi-fi network could take hours.”
Last week Calgary Scientific announced plans to offer Resolution MD through Sprint to provide the app to physicians who own HTC EVO 4G devices. The HTC EVO 4G, which launched in June, is the first phone Sprint has offered for its new "4G" network. Calgary Scientific has also partnered with Siemens, Viatronix and Sentinelle Medical to distribute Resolution MD.
"One of the largest concerns is that data stored on laptops or hand-held devices could be easily copied, putting security and patient confidentiality at risk. PureWeb, the underlying technology, uses advanced architecture that doesn't require image data or confidential patient information to be transferred to the hand-held device. Patient or DICOM data is never removed from the on-site premises; it remains "in the cloud." If an HTC EVO 4G or hand-held device is lost or stolen, the patient records and data remain safe," Sprint and Calgary Scientific explained in a recent press release.
For more, read this report over at Technology for Doctors
Read this press release from Sprint
















