 Houston, Texas-based medical device company Saranas has raised $4M to support continued development on their sensor-enabled bleeding detection device intended for use during cardiac procedures.
Houston, Texas-based medical device company Saranas has raised $4M to support continued development on their sensor-enabled bleeding detection device intended for use during cardiac procedures.
Of the more than 20 million people in the United States who undergo vascular access procedures – where the femoral artery is used to get to the heart – about 1 million of them experience bleeding complications. At first, these bleeds are unseen, and can go completely undetected until the patient begins experiencing other outward symptoms. At that point, the situation can escalate to the point where getting it under control is far more difficult or even impossible, effectively increasing the risk of death and driving up healthcare costs and time spent in the hospital.
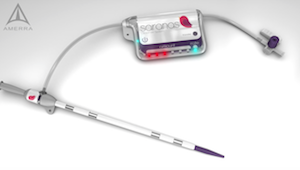
That’s the problem Saranas’ Early Bird bleed detection device is trying to avoid. The technology, which was invented at the Texas Heart Institute, consists of a vascular access sheath embedded with sensors to allow for real-time detection of bleeding caused by accidental rupture of a blood vessel during procedures such as a transcatheter aortic valve replacement. The device is still in the product testing phase, and Saranas plans to submit for FDA clearance later this year.
“The Early Bird system is potentially an important medical advancement for patients who can be affected by serious bleeding complications during cardiac catheterization procedures,” Saranas President and CEO Zaffer Syed said in a statement. “This new investor infusion is a strong vote of confidence in the progress of our technology. With this funding, we expect to complete product development, seek regulatory clearance in the United States and Europe and initiate a clinical pilot with physicians.”


















