 According to a new survey by Validic of 166 executives at pharma and biotech companies, contract research organizations, and others in the clinical trial space, 64 percent have used digital technologies in clinical trials, and 97 percent plan to in the next five years. The survey was presented at DPharm Disruptive Innovations US in Boston.
According to a new survey by Validic of 166 executives at pharma and biotech companies, contract research organizations, and others in the clinical trial space, 64 percent have used digital technologies in clinical trials, and 97 percent plan to in the next five years. The survey was presented at DPharm Disruptive Innovations US in Boston.
"The use of digital health technologies and patient-generated health data is becoming a proven necessity to the operational efficiency and patient-centricity of the pharmaceutical industry," said Validic CEO and Co-Founder Drew Schiller. "This remotely-collected data has the potential to reduce patient burden allowing for more passive engagement and data collection, while also improving the accuracy of data patients contribute during and after trials."
The survey, conducted by technology company Validic, found that 33 percent of respondents had used wearable activity trackers, 36 percent had used sensors such as sensor-enabled pill bottles or ingestible sensors, 45 percent had used in-home clinical grade medical diagnostic devices, and 47 percent had used apps. Wearables and sensors were the areas with the most growth potential, as 67 and 64 percent of respondents, respectively, said they planned to use them in the future.
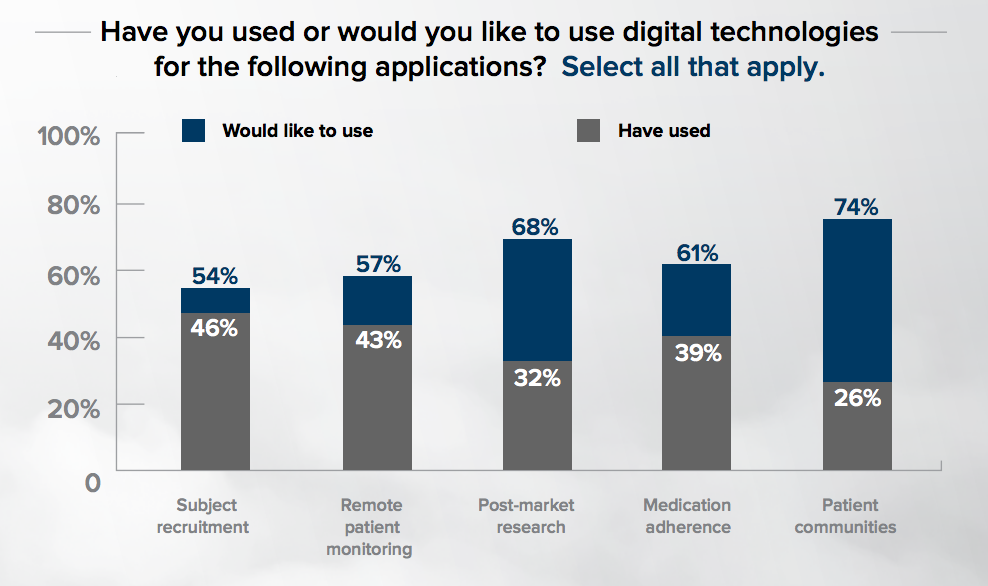
Respondents also differed in how they used digital health technology in trials. Forty-six percent had used digital technology for recruitment, 43 percent for remote patient monitoring, 32 percent for post-market research, 39 percent to improve medication adherence, and 26 percent used technology to support patients in community with one another. The latter area had the most projected growth, with 74 percent hoping to implement technology for patient communities in the future.
Interestingly, the end point companies were most likely to measure with digital tools was medication adherence, followed by activity, heart rate, blood pressure, and sleep. Respondents cited chronic conditions as the area in which they thought digital tools could have the most effect, followed by cardiovascular disease and metabolic disorders.
Validic also asked companies about their business cases for using digital technologies. Seventy-three percent said that digital technology helped to demonstrate the real-world value of an intervention. Sixty-eight percent were motivated by reducing the cost of clinical trials and 68 percent were interested in increasing patient centricity (respondents were instructed to check all that applied).
"Wearable sensors, remote monitoring devices, and mobile apps all have great potential to help us understand how patients are responding to therapies," Kara Dennis, managing director of mobile health at Medidata, a tech company that works with clinical trial vendors and also partners with Validic, said in a statement. "We're excited to be working through the operational and regulatory challenges with clinical trial sponsors, and to be guiding the industry through an intensive phase of exploration and implementation that will define the future of clinical research."
















