 San Diego-based Thrive Feeding has raised a $500,000 seed round to develop smart baby feeding products.
San Diego-based Thrive Feeding has raised a $500,000 seed round to develop smart baby feeding products.
Thrive Feeding Cofounder Brian Wadsworth told MobiHealthNews that four years ago he knew next to nothing about baby feeding until he met a researcher who had devoted her professional career to studying the feeding of babies.
“It was astonishing to me the scale and the scope of the feeding problem of babies,” Wadsworth said. “It’s not in the news, it’s not in the media, no one is writing about it. It’s under the radar. It also astonishes me that no one is doing anything about it.”
For the next few years, Wadsworth researched baby feeding on and off, before eventually founding Thrive Feeding last summer with Ken Smith.
“The story of Thrive was that we came into it knowing we could make a newborn feeding device that would be efficient, simple, and intelligent and would actually tell mothers how healthy their baby was from a feeding perspective,” he said. “There is nothing available to the healthcare system or to mothers themselves that directly tells them that their baby is a heathy feeder. Or if it’s not a healthy feeder, gives any clues as to why not.”
The standard of care today is tracking a baby's feeding indirectly by keeping track of weight gain or loss after birth.
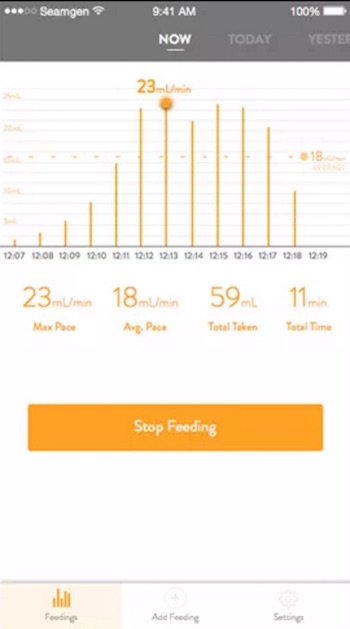
Thrive’s first offering is a baby bottle that will track how much milk a baby drinks from birth to between 60 and 90 days and send data to a companion app. Although Wadsworth said that he is not ready to explain his product in detail — it’s still under development — he said the philosophy of the product is to treat feeding as a performance issue. And to be able to evaluate, measure, track, and record the feeding performance of the baby.
While Wadsworth said the product was under development, he also expects it to be available direct-to-consumer within three to six months.
“When it is born we do not know whether it can adequately feed by mouth and we really want that baby to feed by mouth because the alternative is to put tubes in it or intravenous drips of nutrition and that’s really bad,” Wadsworth said. “The first thing that this technology will do is a definitive screening of feeding ability.”
One future use case for the bottle is with premature babies who are given a feeding tube when clinicians think the baby is having trouble with a bottle. The device would generate new data to help them make that decision, but these types of use cases would require further study and FDA clearances.
The initial plan is for the device to be made available direct-to-consumer to help parents track their baby's progress, Wadsworth said.
“This technology would be used to identify lactation problems that are caused by the infant,” Wadsworth said. “In other words if we know that the infant is a strong feeder from our technology and yet it is not feeding well, we can start looking at the mother or we can start looking at the environment.”
Last October, Atlanta, Georgia-based, NFANT Labs, formerly known as CCB Research Group, received FDA clearance for a smart baby bottle, which uses sensors to measure a baby's tongue strength and sends the data to a provider's mobile device.

















