 Amcom Software announced this week FDA 510(k) clearance for its Amcom (Commtech) Messenger middleware as a class II medical device intended for hospital use.
Amcom Software announced this week FDA 510(k) clearance for its Amcom (Commtech) Messenger middleware as a class II medical device intended for hospital use.
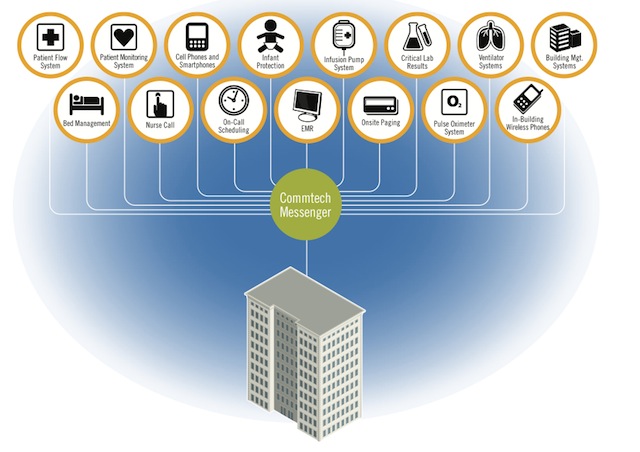
The Amcom Messenger middleware sends critical secondary notifications from patient monitoring and other alert systems to staff carrying wireless communication devices, including smartphones, pagers, and Wi-Fi phones.
"Systems like this -- that pass alerts from clinical systems to caregivers on mobile devices -- were not required to get the OK from the FDA until its February 15th ruling," Amcom VP Mike Devine told MobiHealthNews in an email. "At that point, Amcom Software started the process and is pleased to announce this milestone."
In mid-February the FDA announced changes to its MDDS rule -- read more in the FDA release from February here.
A few weeks after that change of rules at the FDA, paging company USA Mobility bought Amcom Software for $163.3 million in cash. The acquisition looked to help USA Mobility modernize its messaging services beyond legacy paging services and into messaging and unified communications.
“Amcom Messenger helps hospitals improve how quickly staff can react to a variety of time-critical events, which in turn helps improve patient safety and outcomes,” stated Chris Heim, President, Amcom Software in this week's press release. “We’re pleased to have received FDA clearance for this solution as it’s an important consideration for our customers today.”
Read the press release about the FDA clearance below.
PRESS RELEASE -- Amcom Software, Inc. today announced that its Amcom (Commtech) Messenger middleware has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) as a class II medical device. For hospitals, this clearance means Amcom Software is ensuring its solution is keeping pace with key industry standards and guidelines.
Amcom Messenger middleware sends critical secondary notifications from patient monitoring and other alert systems to staff carrying wireless communication devices. Its purpose is to help hospitals improve patient care by supplementing a monitoring system’s audible notifications with targeted messages to the right caregiver.
“Amcom Messenger helps hospitals improve how quickly staff can react to a variety of time-critical events, which in turn helps improve patient safety and outcomes,” said Chris Heim, President, Amcom Software. “We’re pleased to have received FDA clearance for this solution as it’s an important consideration for our customers today.”
Amcom Messenger middleware helps organizations create an enterprise-wide hub to manage, prioritize, and respond quickly to key events. This includes the ability to send messages to the right people on the right device based on rules set up in a given facility. Staff devices can include smartphones, pagers, Wi-Fi phones, and others, enabling hospitals to incorporate their specific needs into their alarm management process.















