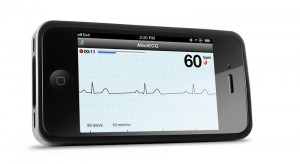
 If there's one device that's recently become a symbol for the promise -- and potential pitfalls -- of mobile health, it's AliveCor Heart Monitor, formerly known as the AliveCor iPhone ECG, which received FDA clearance for prescription use in December. Dr. Dave Albert, AliveCor's chief medical officer, outlined some next moves for the company recently in response to an online conversation about the device.
If there's one device that's recently become a symbol for the promise -- and potential pitfalls -- of mobile health, it's AliveCor Heart Monitor, formerly known as the AliveCor iPhone ECG, which received FDA clearance for prescription use in December. Dr. Dave Albert, AliveCor's chief medical officer, outlined some next moves for the company recently in response to an online conversation about the device.
The AliveCor Heart Monitor has a lot of potential for detecting and gathering data on asymptomatic arrhythmias, which is difficult to do in a clinical setting. But how does a prescribable home-use device fit into a physician's workflow? What are a doctor's responsibilities and liabilities?
Dr. Westby Fisher, an internist and cardiologist who blogs under the name "Dr. Wes," wrote a must-read post last week elucidating some of these questions in the context of the AliveCor device. The post spurred a flurry of comments, comprising a microcosm of the ongoing digital health debate about mobile health, patient rights, and doctor availability.
"Once a doctor 'prescribes' such a device, what are his responsibilities?" Fisher writes. "Does this obligate the physician to 24/7/365 availability for EKG interpretations? How are HIPAA-compliant tracings sent between doctor and patient? How are the tracings and medical care documented in the (electronic) medical record? What are the legal risks to the doctor if the patient transmits OTHER patient's EKG's to OTHER people, non-securely? At this point, no one knows. We are entering into new, uncharted medicolegal territory."
Fisher points out that according to an email he received from the company, AliveCor doesn't currently "provide any ECG interpretation, diagnosis or analysis of the data obtained with the monitor." The email goes on to say that "[p]atients will be instructed to contact you, their physician, regarding any questions they may have regarding their recordings." Answering these questions, whether by phone or secure emails, Fisher points out, would not constitute billable hours, and physicians might not be confident enough in the capacity of a home-administered single-lead EKG to deliver a diagnosis for which they would be legally liable, especially in emergency situations.
In the comments, a debate ensued about whether the device was even valuable, with some healthcare professionals arguing that EKG data, without the expertise to interpret it, would be harmful to patients. A number of heart patients, including noted ePatient Hugo Campos, argued the contrary, citing their own examples of how AliveCor and similar devices had helped them address heart problems, and also suggesting that reading an EKG is not as difficult as some suggest. Many suggested that the root of all the problems Fisher mentioned is that the device is prescription at all.
"The problem here is exactly the requirement that all patients obtain a prescription from a physician before ordering the AliveCor heart monitor," Campos wrote. "Get rid of the prescription prerequisite and you get rid of the problem altogether." He pointed out that the company plans to offer the device in Europe over the counter.
Perhaps most notably, AliveCor inventor and co-founder Albert chimed in, responding to comments and giving some hints about the future of AliveCor. Albert reaffirmed what MobiHealthNews reported in December, that the over-the-counter version of the device is still coming, pending FDA clearance. He also said that the lack of interpretation for results is temporary, based on their planned offerings. The company plans to offer both automated and human interpretation services directly to consumers, on a sliding fee scale, noting that their atrial fibrillation detection algorithm had a 100 percent sensitivity and 96 percent specificity in a trial recently presented at the American Heart Association.
Albert said that having different confidence levels of reads for different prices is part of a disruption he thinks is crucial in medicine.
"[P]eople have to buy the case and will have to pay for the reads -- that will make them think about using it appropriately," he wrote. "Personal financial feedback has been missing in medicine but it is in every other part of our life. Open loop anything is bad for controlling costs and medicine is a prime example of that."
Although the release of an over the counter version and interpretation services might curtail the problems for AliveCor, the concerns about physician workflow Fisher brought up will continue to be present in other prescription remote monitoring devices that follow AliveCor's lead. The conversation isn't going away any time soon, and would-be device developers would do well to keep it in mind.
In a recent interview with MobiHealthNews, Campos elaborated on his belief that anyone can learn to read an EKG, and everyone should be given the chance to do so.
"We often underestimate peoples' ability to make sense of the information," he said. "Atrial fibrillation is not that hard to recognize. The hallmark of atrial fibrillation is an irregular heartbeat. I can recognize that and I never went to medical school. This is the kind of stuff we all think belongs to the physician but there are some things that people can know and do know. We need to unlock the potential that lies in these devices and their ability to democratize this data -- we need to stop being so fearful and be a little more brave, but no one seems to be brave enough to take some risks."


















