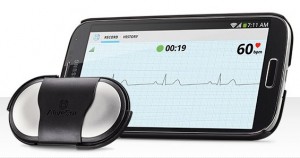
 AliveCor, maker of a low-cost, smartphone-based ECG device for the iPhone, has gained FDA 510(k) clearance for a long-anticipated universal ECG attachment that works with just about any late-model Android or iOS smartphone or tablet, and is now available for sale in the US.
AliveCor, maker of a low-cost, smartphone-based ECG device for the iPhone, has gained FDA 510(k) clearance for a long-anticipated universal ECG attachment that works with just about any late-model Android or iOS smartphone or tablet, and is now available for sale in the US.
The new AliveCor Heart Monitor, introduced Friday at the University of Southern California's seventh annual Body Computing Conference in Los Angeles, is not a phone case like the existing AliveECG for the iPhone or Samsung Galaxy S4, but rather a pocket-size wireless sensor that fits into a plate that attaches to the back of a mobile device.
"The new form factor can be used with your current case," AliveCor founder and CMO Dr. David Albert, an Oklahoma City cardiologist, told MobiHealthNews at the conference. "It gets us out of the case business."
The AliveCor Heart monitor detaches from the plate, connecting with a smartphone not by Bluetooth but with a proprietary, patented ultrasound emission that the phone's microphone picks up. Albert said it consumes one-tenth the power of Bluetooth low energy, saving phone battery life and making it suitable for use in low-resource environments.
The new version, which has FDA 510(k) clearance for sale as a Class II medical device, will retail for $199, just like the AliveECG.
The launch coincides with the opening of a study with Dr. Leslie Saxon, executive director of the Center for Body Computing and chief of cardiovascular medicine at the USC Keck School of Medicine, about how the device might be applied to daily activities. AliveCor is handing out 300 Heart Monitor sensors to conference attendees in exchange for them participating in the 12-month study, which will include as many as 5,000 subjects, according to USC.
The study is a follow-up to initial, small-scale research Saxon had published in the Journal of the American College of Cardiology in March 2012.
San Francisco-based AliveCor does continue to sell the case-like AliveECG – formerly the iPhone ECG – for the Galaxy S4 and for the iPhone 4, 4S, and 5. But the new Heart Monitor is compatible with many other devices, including the fifth-generation iPod Touch. The company promises support for the new iOS 7 – and, by extension, the iPhone 5c and 5s – in the near future.
AliveCor is currently marketing the new product for the high-end Samsung Galaxy S3, S4, and HTC One and several iPhone models, but Albert said the Heart Monitor will work with many other Android phones and tablets. "There are markets like India and China where low-cost Android phones are dominant," Albert said.
For now, however, AliveCor is only offering its mobile ECGs in the US and UK. While the AliveECG does have the CE Mark, allowing sales across the European Union, and translation engines are available, Albert said he wanted to concentrate on English-speaking markets for now.
AliveCor has had some management turmoil of late, appointing new a CEO in August for the second time in a year. New CEO Euan Thomson, operating partner of AliveCor investor Khosla Ventures, spoke Friday morning at the Body Computing Conference, but his session focused on mobile cloud computing.


















