 Great Lakes NeuroTechnologies, a company using tablets and wearable sensors for Parkinson's diagnosis and therapy, will use a recent $1.5 million NIH grant to start a move toward direct-to-consumer marketability. This is the latest in a long series of NIH grants for the company since 2005 and brings the company's total grants to $14.2 million.
Great Lakes NeuroTechnologies, a company using tablets and wearable sensors for Parkinson's diagnosis and therapy, will use a recent $1.5 million NIH grant to start a move toward direct-to-consumer marketability. This is the latest in a long series of NIH grants for the company since 2005 and brings the company's total grants to $14.2 million.
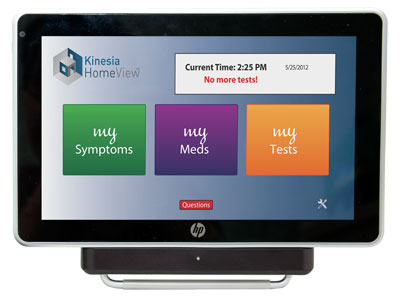
The system, called Kinesia HomeView, is currently sold in the clinical trial market and requires GLNT to provide users with an HP Slate tablet, a broadband connection, and the company's Bluetooth-connected sensor, worn on the fingertip.
"Currently the majority of our market is made up of selling our technology into the clinical trial space: To pharmaceutical companies, companies that manufacture deep brain stimulation. We sell our devices into those clinical trials," Joe Giuffrida, GLNT president and principal investigator, told MobiHealthNews. "That type of market can tolerate the price point of the technology with the tablet and with the broadband connectivity charges. However, to get into the consumer market or the patient care market, we have to get into mobile applications to reduce the cost for those markets."
Kinesia HomeView users will still have to get the fingertip sensor from the company, however. Giuffrida said the smartphone's built-in sensors were not sensitive enough to give useful and accurate information about Parkinson's disease.
 "If you use the accelerometer in a smartphone, you can get general actigraphy information — how active of a person are they, are they sitting down, walking around," he said. "However, if you want to actually collect Parkinson’s symptoms such as what’s going on with a tremor in the hand or gait or dyskinesia or bradykinesia, the sensors have to be in a certain place on the body in order to detect those finer resolution of symptoms."
"If you use the accelerometer in a smartphone, you can get general actigraphy information — how active of a person are they, are they sitting down, walking around," he said. "However, if you want to actually collect Parkinson’s symptoms such as what’s going on with a tremor in the hand or gait or dyskinesia or bradykinesia, the sensors have to be in a certain place on the body in order to detect those finer resolution of symptoms."
Giuffrida said the difficulty in moving to an app-based system will be in making sure the user interface is still intuitive.
"One of the biggest challenges is going to be to really simplify the interface for the user," he said. "When you’re moving to a smaller platform there’s a lot of design consideration and workflow — especially when you’re dealing with someone who has Parkinson’s and has movement disorder symptoms to begin with —to make sure they’re going to be able to use a smaller form factor."
The company plans to have the mobile app completed by the end of the year. The grant will also help the company continue to develop algorithms for detecting and measuring bradykinesia, a Parkinson's symptom characterized by frozen joints and slowed movements.
As we reported back in 2011, the tablet (and soon, the mobile app) presents patients with video instructions on taking the Unified Parkinson’s Disease Rating Scale tests. It records them taking the test several times a day with both the device's camera and the sensors. Since then, GLNT has doubled its team, secured a number of patents and has expanded its development from Parkinson's diagnostics into some early trials of Parkinson's therapy. For instance, the company recently completed a trial of transcranial direct current stimulation — delivering electric currents to sleeping patients with the goal of improving their mobility when they wake.

















