 MobiHealthNews has been tracking FDA clearances for smartphone-connected medical devices and standalone apps for many years. So far 2014 has had its fair share -- about two dozen digital health-related FDA clearances have been secured this year. Here's a roundup:
MobiHealthNews has been tracking FDA clearances for smartphone-connected medical devices and standalone apps for many years. So far 2014 has had its fair share -- about two dozen digital health-related FDA clearances have been secured this year. Here's a roundup:
New York City-based Kinsa Health received FDA 510(k) clearance for its Kinsa smart thermometer, which can be used, orally, under-arm, or rectally. The device transmits data to a companion smartphone app.
Samsung’s S Health app received 510(k) clearance from the FDA as a cardiology signal transmitter suggests that the clearance will allow S Health to interface with additional connected medical devices in the United States.
Waltham, Massachusetts-based EarlySense, which makes a passive and contactless bedside monitor that continuously measures respiration rate, heart rate and motion, has received a new FDA clearance for a sensor designed to work with not a bed, but a chair.
Alere Connect, formerly known as MedApps before it was acquired by Alere, announced FDA clearance for its latest home health hub, HomeLink.
A British company, Camntech, has received FDA clearance for a motion-tracking wristband and a wristworn electronic diary, likely for use in clinical trials. The devices are called the MotionWatch 8 and PRO-Diary.
Carestream received FDA clearance for VUE Motion, a mobile radiology viewing and reading software that can be accessed on iPhone 4s, iPad 2, Galaxy Note and Galaxy S III mobile devices.
Remote patient monitoring company HealthInterlink received FDA 510(k) class II clearance for Beacon 2.0, a mobile-centric software system that integrates data from various home health devices. Beacon was previously classified as a class I medical device (MDDS).
The FDA disclosed 510(k) clearances for about a dozen digital health apps and devices during the second quarter of the year.
 Propeller Health, formerly Asthmapolis, received FDA clearance for a platform that includes a new smart inhaler and is geared for patients with either asthma or COPD.
Propeller Health, formerly Asthmapolis, received FDA clearance for a platform that includes a new smart inhaler and is geared for patients with either asthma or COPD.
In June San Francisco-based Qardio received FDA 510(k) clearance for its connected blood pressure monitor, called QardioArm. The device went on sales for $99 on Qardio’s website and in select stores the following week.
New Zealand-based medical device company Nexus6 received FDA clearance for its smartphone-connected inhaler, SmartTouch, as a class II medical device. The new SmartTouch device has been cleared as a prescribable Metered Dose Inhaler (MDI) with a handful of intended uses: in clinical trials; in clinical practice, and for patient self-management.
Atlanta, Georgia-based health device maker CardioMEMS received FDA clearance for its CardioMEMS HF System, which monitors pulmonary artery pressure. The clearance was only for patients who have experienced New York Heart Association (NYHA) Class III heart failure and have been hospitalized for heart failure in the previous year.
Alberta, Canada-based Calgary Scientific announced that it received a new FDA clearance for its diagnostic medical imaging software, called ResolutionMD, that enables providers to use the mobile software for all imaging modalities, except mammography.
McKesson secured clearance for a mobile medical app called McKesson Cardiology ECG Mobile. The web-based version of McKesson Cardiology ECG has been around for a few years and it enables clinicians to analyze and review ECG waveforms captured by a variety of vendors’ ECG devices.
 InTouch Health received clearance for an app that would allow auscultation from digital stethoscopes in near-realtime. InTouch says that up until now digital stethoscopes have relied on store and forward technology, but InTouch’s CS App transmits live from a patient to a doctor at a remote location.
InTouch Health received clearance for an app that would allow auscultation from digital stethoscopes in near-realtime. InTouch says that up until now digital stethoscopes have relied on store and forward technology, but InTouch’s CS App transmits live from a patient to a doctor at a remote location.
Reflectance Medical secured a 510(k) clearance for a tablet-based version of its Multi-Parameter Mobile CareGuide 3100 Oximeter system. The original device offers a “non-invasive assessment of hemoglobin oxygen saturation and pH in a region of skeletal muscle tissues beneath the oximeter sensor,” according to the company.
Vital Connect received FDA clearance for its Vital Connect Platform, which is the system that supports the company’s peel-and-stick, Bandaid-like vital signs monitor HealthPatch. The wearable device captures single lead ECG, heart rate, HRV, respiratory rate, skin temperature, body posturing (fall detection), steps, stress, and sleep staging. The company also received FDA clearance for the system's use in the home.
Gauss Surgical announced that it has received de novo FDA clearance for an app that uses the iPad’s camera to estimate the amount of blood lost during a surgery and captured with surgical sponges. The system is called the Triton Fluid Management System.
Entra Health Systems, one of the earliest entrants into the smartphone-connected glucometer space, received clearance for the MyHealthPoint telemedicine manager, an online and mobile software offering that would collect and store biometric data from a variety of sources. It measures vitals including glucose, blood pressure, weight, body composition, activity, body temperature, ECG, and pulse oximetry.
So far, the third quarter of the year has produced a handful of FDA clearances for digital health devices and apps, too.
 Another clinically-focused, activity tracking wearable has now been cleared by the FDA, this one aimed specifically at the monitoring and treatment of Parkinson’s disease. The Personal KinetiGraph, from Melbourne, Australia-based Global Kinetics Corporation, “offers comprehensive, automated reporting of a Parkinson’s disease patient’s movements so that neurologists and other physicians can more easily identify changes in movement symptoms to assist in decisions to optimize therapy,” according to the company.
Another clinically-focused, activity tracking wearable has now been cleared by the FDA, this one aimed specifically at the monitoring and treatment of Parkinson’s disease. The Personal KinetiGraph, from Melbourne, Australia-based Global Kinetics Corporation, “offers comprehensive, automated reporting of a Parkinson’s disease patient’s movements so that neurologists and other physicians can more easily identify changes in movement symptoms to assist in decisions to optimize therapy,” according to the company.
Otoharmonics, a startup out of the Baker Group supported by Cedars-Sinai Medical Center, received FDA 510(k) clearance for an iPad or iPod Touch application that treats a medical condition called tinnitus.
Australian company dorsaVi received FDA clearance for its ViMove sensor system. The sensor tracks movement as well as muscle activation, and is intended to be used in a clinical setting or with athletes in training.
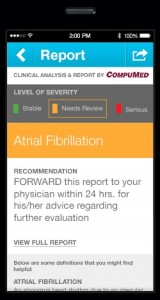
AliveCor has received an additional FDA 510(k) clearance, this time for an algorithm that allows its smartphone ECG to detect atrial fibrillation — an abnormal heart rhythm that isn’t always detectable to the patient, but if left untreated can lead to stroke or congestive heart failure — with high accuracy.
New York City-based medical device maker Philosys received FDA 510(k) clearance this week for its smartphone-connected glucose meter, Gmate Smart.


















