 A smart baby bottle, which uses sensors to measure a baby's tongue strength and sends the data to a provider's mobile device, has received FDA clearance. Atlanta, Georgia-based NFANT Labs, formerly known as CCB Research Group, has been working on the system for about a little under two years.
A smart baby bottle, which uses sensors to measure a baby's tongue strength and sends the data to a provider's mobile device, has received FDA clearance. Atlanta, Georgia-based NFANT Labs, formerly known as CCB Research Group, has been working on the system for about a little under two years.
"Many infants in the NICU have trouble transitioning from tube feeding to bottle or breast feeding and these problems often lead to delays in patient discharge,” Dr. Gilson Capilouto, professor in the College of Health Sciences at the University of Kentucky and co-founder of NFANT Labs, said in a statement. “Our product focuses on tongue movement since the tongue plays a major role in safe swallow during feeding. Like any muscle, it’s equipped with strength properties that can be measured and rehabilitated. Through nfant Feeding Solution we have created the first and only medical device that can determine an infant’s tongue movement during feeding and offer a method to capture and return data to the caregiver.”
According to an interview last year with BusinessLexington, the status quo in measuring whether an infant has developed the necessary strength to switch to bottle or breastfeeding is a subjective test where the doctor puts his or her finger in the baby's mouth and makes a judgement call. The nfant system represents the first attempt to introduce hard data into the equation. The company will sell the system to hospitals to use in their neonatal intensive care units (NICUs).
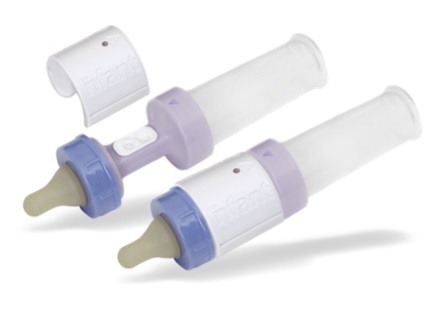
The system consists of a disposable Coupling device that sits between the nipple of the bottle with the rest of it. A reusable sensor is attached to the Coupling device to non-invasively measure the infant's tongue strength, and then send that data via low energy Bluetooth to the caregiver. It can be used either for a one-time assessment, or to track the trend of an infant's feeding habits.
“Up to 70 percent of the 540,000 premature babies born in the US each year experience feeding problems," Lou Malice, CEO of NFANT Labs, said in a statement. "Until now, physicians and caregivers have not had access to objective feeding data and the power of cloud analytics to enhance decision making.”
The device received FDA 510(k) clearance as a class 2 medical device for prescription use only. The predicate device, the NTrainer system from Innara Health, incorporated similar sensors into a pacifier-like device, which means it couldn't be used on infants who were actually feeding, only those engaged in what's referred to as "non-nutritive sucking."

















