 Roche has selected two health technology companies to deploy a wireless wearable system in a clinical trial on a drug for infants with spinal muscular atrophy.
Roche has selected two health technology companies to deploy a wireless wearable system in a clinical trial on a drug for infants with spinal muscular atrophy.
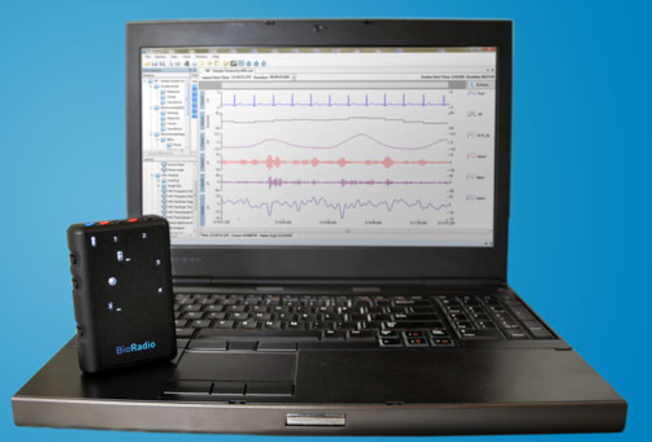
The study will use BioRadio, a wireless wearable data acquisition system from Great Lakes NeuroTechnologies that records respiratory and pulmonary data from infants in near real time. Vivonoetics’ VivoSense software works in collaboration with BioRadio to provide data analysis. The device will measure patterns of movement of the chest and abdominal wall, allowing for objective measures of pulmonary function.
Conducting clinical studies on infants comes with a special set of challenges, particularly since devices that can be used in adults, like respiratory-measuring masks and mouthpieces, just may not translate to use in tiny babies. As such, studies on spinal muscular atrophy – wherein weak respiratory muscles hinder breathing ability – have been compromised by their inability to accurately gather the necessary longitudinal measurements to develop targeted interventions. So Roche is working around that by tapping the non-invasive BioRadio and VivoSense system.
“Great Lakes NeuroTech is very proud to collaborate with Vivonoetics on this exciting study in SMA. This represents successful integration of wearable technology and advanced data analytics to capture infant respiration.” Carissa Simmerman, BioRadio Clinical Trials Manager at Great Lakes NeuroTech said in a statement. “We are strongly committed to using wearable and remote monitoring technology to make a positive impact on quality of life for those affected by respiratory disorders.”
Great Lakes NeuroTechnologies said the study will be the first large-scale spinal muscular atrophy clinical trial to use the RP technology, and the BioRadio device has also been used in a clinical trial at Boston Children’s Hospital to monitor the impact of stress on children. Another clinical trial targeting therapeutic interventions for children with Rett syndrome is also using the device.
BioRadio works by using Respiratory Inductance Plethysmography (RP)-sensing technology, and the device can capture many physiological signals including heart activity, brain waves, muscle activity and pulse oximetry. Vivonoetics was chosen as a collaborator for its reputation as a leader in RP analysis in clinical trials, and the proprietary VivoSense software is expected to enable analysis of clinically significant changes in respiratory morbidity and mortality.
“We strive to advance clinical trials through the use of wearable technology,” Vivonoetics COO Dudley Tabakin said in a statement. “With our expertise in wearable respiratory sensors and detailed data analytics we have developed a solution that works for monitoring infants with airway obstruction observed in SMA.”


















