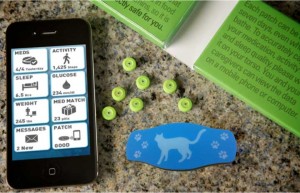
Proteus Biomedical's Raisin system
Proteus Biomedical raised about $17.5 million in a round of funding it hopes will eventually top $50 million, according to a regulatory filing. The Redwood, California-based company developed an "intelligent medicine" suite of technology called Raisin, which includes, an ingestible biomedical sensor, a wearable, peel-and-stick patch, and a companion smartphone app. The technology has been piloted by Novartis, the UK's NHS, a clinic in New York, and other less public partners. A pharmacy chain in the UK was the first to rollout a service offering with components of the Raisin platform earlier this year.
It has been a few years since Proteus raised money.
In 2010 the company announced that Novartis had invested $24 million in the company as part of an exclusive deal that leveraged Proteus ingestible technology with patients taking organ transplantation medications. While Novartis mentioned plans soon after to help push regulatory clearance along for the technology, nothing more has come out about the partnership.
Shortly before that at the end of 2009 Proteus quietly raised about $25 million in funding.
The companies first major product launch went live earlier this year: UK-based retail pharmacy chain Lloydspharmacy inked an exclusive deal with Proteus Biomedical to launch Proteus’ first commercial product, Helius. The offering is based on Proteus' Raisin technology, and it includes sensor-enabled pills, a peel-and-stick sensor patch worn on the body, and a mobile health app. The patch records when a pill is ingested, tracks sleep patterns, and records physical activity levels. Proteus’ Raisin system secured a CE Mark last year and it is expected to roll out commercially in other countries across Europe soon, too. Given that the UK's NHS announced plans to pilot Proteus' ingestible sensors in mid-2010, it was little surprise the company launched in that country.
Lloyds will offer the Helius system as part of a personalized, medication adherence pack to its customers, which its pharmacists assemble for each individual customer. (This is a common practice in the UK and other parts of the world, but not so much in the US.) For example, if a person takes five medications each morning, one blister pack would include all five of those medication, but it will also include a Helius tablet, which has an embedded sensor to track when it is ingested. That way, the Helius system can track when a person takes each group of pills in their drug regimen. If there are three groups of pills to take morning, noon, and night — the Helius packs include a sensor-enabled tablet with each group.
At the beginning of this year, BodyMedia announced plans for a disposable, peel-and-stick fitness sensor that was built using Proteus technology. Last summer Proteus announced that it had secured another patent for its ingestible biomedical sensor. In early 2010 the company received FDA clearance for its wearable patch technology, but the ingestible sensor still does not have FDA clearance. In 2009 Proteus CEO Andrew Thompson had a great talk at TEDMED, which MobiHealthNews attended and reported on live (more here), where he predicted that "iMeds" would be bigger than iTunes.
Thomspon has long touted the company's prospects: Three years ago I was on a panel with him and at the time he estimated that his company’s market opportunity was $100 billion.














