An iPhone app and peripheral device that allows doctors to use the iPhone camera to take photographs of the interior surface of the eye has received 510(K) FDA clearance.
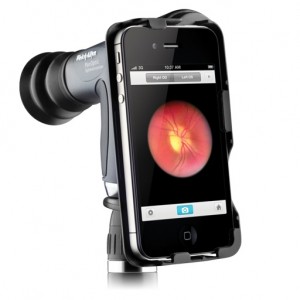 The iExaminer System from Welch Allyn will build on the company's existing PanOptic Opthalmoscope, a device that lets a physician see into the back of a patient's eye. The FDA clearance is for an adapter that connects the opthalmoscope to an iPhone 4 or 4s and an app that will allow the iPhone to take about 85 images in five seconds.
The iExaminer System from Welch Allyn will build on the company's existing PanOptic Opthalmoscope, a device that lets a physician see into the back of a patient's eye. The FDA clearance is for an adapter that connects the opthalmoscope to an iPhone 4 or 4s and an app that will allow the iPhone to take about 85 images in five seconds.
"It really is more of an enhancement or supplement," said product manager Rick Farchione. "It provides the ability to store and analyze the image, send for consultation, and save for future comparison. That's what it does better than a traditional opthalmoscope."
Farchione said the primary market for the device, which is cleared for medical professional (prescription) use only, is not eye specialists. The goal is, instead, to give primary care physicians and multi-specialist clinicians the means to do an eye exam without more expensive equipment (the whole system costs about $600). Images of the interior of the eye, or the fundus, can help doctors diagnose glaucoma, hypertension, and papilledema. Perhaps the most common case, Farchione said, is diabetes. He said about half of diabetes patients don't get an eye exam when they should.
With the pictures instantly available on the iPhone screen, doctors can easily show the patient a picture of their eye, which can be helpful in motivating a patient to seek treatment.
The MIT Media Lab has developed technology that uses an iPhone to detect cataracts and a consumer-facing eye diagnostic peripheral, but neither tool fills the same role as a fundus camera. Farchione said it's unlikely the company will adapt this technology for consumer use.
Currently the FDA clearance is only for the iPhone 4 and 4s, and Farchione said the device is not currently designed to work with the iPhone 5.
"Not to say we will not do it," he said, "but we don't have definitive plans at this time."













