 iDoc24 has launched a dermatology question and answer app, First Derm, which is specifically targeted towards mothers to use for their children.
iDoc24 has launched a dermatology question and answer app, First Derm, which is specifically targeted towards mothers to use for their children.
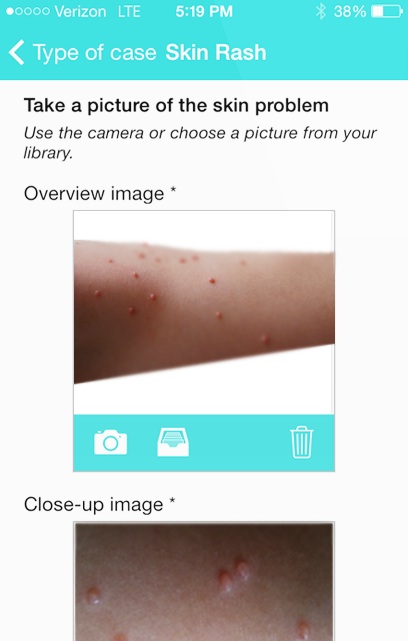
The app allows users to anonymously take pictures of external skin problems and send them to a licensed dermatologist, who will respond to inquiries within 24 hours of receiving the pictures with an assessment of the problem. Users must pay $39.99 for each case submitted for assessment, but for the first three months of the product's launch, users only pay $15 to submit a case.
Users will not have to make an account or register to use the app. First Derm aims to keep all user information anonymous. Another addition to the app is its geo-location abilities that allow a user to locate the nearest pediatrician, dermatologist or pharmacy.
All dermatologists are associated with iDoc24, which offers a variety of mobile apps including STD Triage, which answers user's questions about STDs and iDoc24 PRO, which is meant for doctor use.
"The main focus is to triage the right patient at the right time to the right level of healthcare," iDoc24 CEO and Founder Dr. Alexander Borve told MobiHealthNews in an email. "We have an international network of dermatologists, working in five different languages. They have been vetted by our advisory board. Around 20 percent of cases are audited every month to keep a high standard."
Borve said the company offers the 24-hour policy because the dermatologists usually answer cases when they have time -- most often on their iPads as they travel to and from work.
The company has been testing its products on a European audience since 2009. Since they started, the company has found 25 percent of queries coming in were on moles, 30 percent were STDs and 45 percent were on-skin rashes. Borve offered the app at different price points when testing it with different audiences -- free, $20, $30, and $40.
First Derm is not reimbursable currently, but Borve hopes that it will be after his company can show insurance companies that office visits are avoidable. His product also has a CE mark, but he will not seek FDA clearance.
"FDA as I understand it is for diagnostics," Borve said. "We are not diagnosing, but merely like a Google search finding information, but we have substituted algorithms with information coming from human experts."
Last year, Borve also conducted a small validation study of 69 lesions. The study found that teledermatology assessments done using an iPhone, a dedicated app, and a connected dermascope, could be almost as effective as a face-to-face dermatology consultation.














