 Proteus Digital Health
Proteus Digital Health
Out of all the digital health stakeholders, pharmaceutical companies have both one of the biggest opportunities and some of the most significant challenges to make the most of mobile and digital technologies. As such, while providers and payors have dived into the digital health world with gusto, moves from pharma have often been tentative. They are just now starting to take off. [Download this in-depth report as an eBook (PDF) right here.]
Challenges for pharma
Pharma is fighting an uphill battle in many ways. One is that, as technologies like mobile devices, social media, and video communications bring healthcare into patients' homes, patient trust becomes more important than ever. And pharmaceutical companies generally aren't as trusted as physicians or even insurers.
A Deloitte survey last year found that although people trust doctors and academic centers most, the next most trusted entities are companies like WebMD, then Google search results. At the bottom of the list is pharma and payor companies.
“So the worst part about that is those groups are the ones providing the largest amount of health information and consumers don’t trust them at all,” Deloitte physician Harry Greenspun told MobiHealthNews at the time. “So one of the most interesting things we’re seeing in health is partnerships with pharma and payors and with other organizations to get that info, and get those apps in a method they can believe in, and trust.”
A more recent study, by Makovsky Health, found that only around 35 percent of consumers would trust a disease website sponsored by a pharmaceutical company, and 16 percent said they would never visit a disease website sponsored by a pharma company. Paulo Machado, a healthcare consultant whose past credits include marketing and innovation roles at AstraZeneca and Bristol-Meyers Squibb, summed up the situation.
"If I’m a consumer do I want to talk to pharma companies or do I want to talk to my doctor? Who am I going to believe more?” he said. “The biggest competitors to pharma are not other pharma companies, it’s other healthcare organizations.”
Another challenge is that pharma is historically extremely risk-averse, which Pharmica consultant Matt Hendricks said could contribute to the fact that so few pharma companies have groups like J&J's Janssen or Merck's M2i2.
 "So, why isn’t it happening? Why doesn’t every major pharmaceutical company have a group like Janssen?" Hendricks asked at a panel discussion last December during HIMSS' mHealth Summit. "Why doesn’t every pharmaceutical company have a booth downstairs? There are some very big impediments that are worthwhile to address. One, is when you look at the development of a drug program, it is really all about managing risk and being able to cross off all the risks associated with developing a drug. When you introduce a new factor, it inherently increases risk. In a business that is focused on reducing risk, then those are things that life sciences companies are going to stay away from."
"So, why isn’t it happening? Why doesn’t every major pharmaceutical company have a group like Janssen?" Hendricks asked at a panel discussion last December during HIMSS' mHealth Summit. "Why doesn’t every pharmaceutical company have a booth downstairs? There are some very big impediments that are worthwhile to address. One, is when you look at the development of a drug program, it is really all about managing risk and being able to cross off all the risks associated with developing a drug. When you introduce a new factor, it inherently increases risk. In a business that is focused on reducing risk, then those are things that life sciences companies are going to stay away from."
Since the digital health opportunity is so new, Hendricks argues pharma companies are more comfortable sticking to what they know and what is proven.
"Even though the economics of developing new drugs are becoming increasingly difficult, at the end of the day it is still a well understood process that the finances are easy to figure out," Hendricks continued. "When comparing an opportunity with so many risks and lack of clarity around regulatory approval and business models to something that has been around and been well-trodden for many decades now and has well-trodden business models, when a company is looking at where to put its resources, it’s going to go to where it believes it can get the highest return. Many of these companies still see the biggest opportunity for their resources is in developing drugs and not in developing other therapies that improve health outcomes but may be riskier."
Looking Beyond the Pill
 Care4Today app from Janssen Healthcare Innovation
Care4Today app from Janssen Healthcare Innovation
Outside of the use of mobile devices by pharma sales reps, pharma's movements in digital health over the past few years have fallen broadly under a few basic umbrellas: patient engagement tools, including medication adherence, prescribable digital therapies, new kinds of sensors, and big data initiatives. Like payers, though, pharma companies aren't limiting themselves to areas that relate directly to their core business of selling drugs and therapies.
"We think that there's a transformation that's waiting to happen," Aman Bhandari, director of Merck innovation group M2i2 recently told MobiHealthNews. "Some people say the work that you're doing, population health, that seems like the work of delivery systems. That seems like the work of payers or plans. You know, ultimately, we see a major convergence happening in terms of goals and outcomes and we see a real systemwide refocusing on values and outcomes measurement as kind of the central theme that's driving everyone across the industry. We think there’s an extraordinary opportunity for pharma to have a seat at the table that it hasn't had before and we're hoping to give it that."
Merck's innovation group is supporting a wide variety of research partnerships in the areas of capturing the patient voice, clinician-facing technologies, and innovative uses of data. Similarly, Johnson & Johnson's Janssen Healthcare Innovation group is working on some things related to pharma -- like medication adherence app Care4Today and mobile-enabled clinical trials -- bit is also working on integrated care business offerings for healthcare, using software to improve rates of cardiac rehabilitation.
As M2i2's Chief Medical Information and Innovation Officer Sachin Jain puts it "the ultimate incentive is that we as a company are gradually finding our way into the outcomes improvement business, as opposed to the pill and vaccine business, and as we do that, I think we realize that data and technology and HIT is going to be a critical enabler."
Jain thinks that as the healthcare system as a whole moves toward outcomes-based medicine, pharma companies are no exception.
"As this space evolves, delivery systems, physicians, pharma companies, device companies, government, payers, employers, they’re all starting to coalesce around the notion of value," he said. "I think that’s going to be the unifying factor for healthcare delivery going forward. … A lot of it has to do with the increasing attention paid to measuring outcomes. Health information technology is enabling us to actually measure real world outcomes. ... There’s vast new stores of data. There’s more data about the use of our products outside our walls than there is inside our walls. So there’s an increasing focus on using that data to better understand the real world impact of drugs."
Merck's partnerships are partly about collecting some of the data that's increasingly available to providers and payors and using it to improve therapeutic products.
 "One of the big problems the healthcare industry needs to solve is this translation gap or implementation gap," he said. "How can we make sure that patients actually get the maximal benefit from the products we so painstakingly develop? Making sure providers have the right information, patients have the right information and the right tools."
"One of the big problems the healthcare industry needs to solve is this translation gap or implementation gap," he said. "How can we make sure that patients actually get the maximal benefit from the products we so painstakingly develop? Making sure providers have the right information, patients have the right information and the right tools."
Medication Adherence
Medication adherence is one focus area where the rewards for pharmaceutical companies are very much self-evident: getting patients to actually take the drugs prescribed to them improves health outcomes, could save the healthcare system an estimated $290 billion per year, and, to the pharma company's benefit, helps sell more drugs.
One of Janssen's biggest mobile efforts is the company's Care4Today Mobile Health Manager, an adherence app that is notably not branded and contains a database of drugs from Johnson & Johnson, from Johnson & Johnson's competitors, and over-the-counter medications and vitamins. Janssen Healthcare Innovation Founding Partner David Tripi told MobiHealthNews that move reflects the fact that improving adherence improve's everyone's sales.
"It took us a lot of work to convince our brands not to brand the application," he said. "A lot of brand teams want their branding. And what we’ve seen to date from our competitors is that they are more focused on the branded application then the more generic one. Will they change and follow the model we’re doing? I don’t know. But at the end of the day, we all have the same goal — to get patients to take the medication prescribed by their doctor which ultimately leads to the best outcomes for them, but secondarily results in them refilling their medications more frequently and leads to increased sales for the company. So even if I were to hear [that our patients are using] a competitor's app... that’s not so bad. Whatever’s helping them, that’s great."
But, he admitted, even without branding there's a benefit to Johnson and Johnson being the one to give that help: data. The app also collects deidentified data about medication adherence habits, including which drugs have the most adherence, for which conditions people are most adherent, and so on. Janssen has plans to start running analytics on that data set that could be fed into Care4Today to improve adherence further, or into drug development.
Other adherence moves by pharma are more focused on particular brands -- a way for companies to protect their investment in a given drug. Smart pill bottle AdhereTech has partnered with Boehringer Ingelheim in the hopes of distributing their pill bottle bundled with cancer drugs, for instance. Pharma company Opko bought smart inhaler company Inspiro Medical in order to distribute its smart inhaler with its asthma drugs.
Prescribable pharma-digital therapies
 While medication adherence is pharma's big digital health opportunity, a key trend that is moving pharma in that direction is the slow but sure rise of prescribable mobile-integrated therapy products. In the future these will likely take the form of a pharmaceutical therapy and a companion digital tool -- whether that be a tracking app, ingestible or wearable sensor, a smartphone-enabled personal health device -- or all three. These integrated pharma-digital therapies will be prescribed by physicians to their patients.
While medication adherence is pharma's big digital health opportunity, a key trend that is moving pharma in that direction is the slow but sure rise of prescribable mobile-integrated therapy products. In the future these will likely take the form of a pharmaceutical therapy and a companion digital tool -- whether that be a tracking app, ingestible or wearable sensor, a smartphone-enabled personal health device -- or all three. These integrated pharma-digital therapies will be prescribed by physicians to their patients.
Already pharmaceutical companies are beginning to work with companies that are developing these kinds of digital health tools.
WellDoc is one company that has actually managed to get their mobile-enabled diabetes management program to be cleared by the FDA and paid for by health insurers as they would a pharmaceutical product.
"These could also have potential for a novel revenue stream. Look at what a company like WellDoc has done. They have taken a product [called BlueStar] and shown clinically that they are able to meet an outcome measure. They take that clinical data and go to a regulatory authority who agrees they are allowed to market a claim. Then they go and get reimbursement to the tune of what some people say is between $50 and $100 per patient, per month," Pharmica's Hendricks explained. "That is a lot different from the 99 cent app model, and something that really could be explored by the pharmaceutical industry at a time when they are facing a record number of drug patent expirations and increasing drug development costs.”
WellDoc's BlueStar is an unusual case today -- no other mobile health program has managed to get similar status to medication in terms of pharmacy adjudication and payer reimbursement. The company's success has led to a recent major $20 million investment that included participation from Merck Global Health Innovation Fund.
While they are not prescribable digital health tools, Sanofi Aventis and Johnson and Johnson (LifeScan) developed iPhone connected glucose meters and companion apps. Sanofi launched the iPhone connected iBGStar glucose meter in early 2012 at Apple Stores and Walgreens. In March 2013 the FDA cleared LifeScan's long awaited diabetes tracking app, which works with one of the company's Bluetooth-enabled OneTouch glucose meters. It's not hard to imagine connected glucose meters like these becoming part of a prescribable kit along with some kind of diabetes medication and even paired with a mobile-based program like WellDoc's BlueStar -- in the not too distant future.
Another company that is developing a technology that will likely be a key prescribable digital health therapy is Proteus Digital Health. The technology in the Proteus digital medicine platform includes unique measurement tools, like sensor-enabled pills, a peel-and-stick biometric sensor patch worn on the body, and companion smartphone apps. The patch records when a pill is ingested and also tracks other things like sleep patterns and physical activity levels. The ingestible sensor component secured FDA clearance in July 2012, while the company’s sensor-laden patch got FDA clearance in 2010. While Proteus has previously announced relationships with pharma companies like Novartis and Otsuka, the company has been relatively quiet recently about its current lineup of pharma customers.
Clinical trials
 The other most obvious area of focus for mobile health in pharma is the clinical trial. Mobile startups are focused on innovating nearly every aspect of the clinical trial including recruitment, informed consent forms, data reporting, and training of investigators. Janssen's Clinical Trial Innovation Unit is working on several different ideas including smart blister packs to track adherence in drug trials. They also led the creation of a shared online databank of all non-proprietary clinical information a standard online portal for investigators to communicate with pharmaceutical companies.
The other most obvious area of focus for mobile health in pharma is the clinical trial. Mobile startups are focused on innovating nearly every aspect of the clinical trial including recruitment, informed consent forms, data reporting, and training of investigators. Janssen's Clinical Trial Innovation Unit is working on several different ideas including smart blister packs to track adherence in drug trials. They also led the creation of a shared online databank of all non-proprietary clinical information a standard online portal for investigators to communicate with pharmaceutical companies.
Companies like Omniscience are focusing on innovating the clinical trial with electronic versions of the information packets participants receive, which are easier to access and harder to lose than paper, or recruitment by automated text message, which presents a lower barrier to entry and can therefore facilitate a more representative trial group.
Pfizer created an all-mobile and online clinical trial in 2011. Pfizer’s study, a clinical trial involving an overactive bladder treatment launched in the summer of 2011, was poised to be the first clinical trial that patients could participate in remotely, with even the drugs delivered to the patients by mail. Previously, Pfizer had conducted a traditional trial on the same drug, Detrol LA. The pilot was focused on testing the online method itself and on promoting patient engagement in the research.
The study was shut down for lack of participation, but successfully retried it in 2013. Lead investigator Miguel Orri said that the biggest hurdles the second time around were regulatory.
"What would help is regulation around the telemedicine laws, which are not designed for clinical trials, but something we need to adhere to in a clinical trial setting," he said. "This is in a way contradictory, because there are prescription laws, which make sense, but they are not relevant to clinical trials. The telemedicine laws, on the other hand, don’t differentiate between a clinical trial or regular setting."
The other barrier for mobile-enabled clinical trials is, again, risk aversion in pharma, which is also related to regulation. Jean-Luc Neptune, now a part of Health 2.0, founded a mobile clinical trial company called Healogica, which shut down in 2010.
"There is this regulatory paralysis that exists in the pharmaceutical industry, and people are literally afraid to make decisions," Neptune said at the time. "I can’t tell you how many times we talked to somebody and they’ve said ‘Hey, I’ve got to talk to my lawyer, and I’ve got to talk to my in-house counsel.’ This is a huge space, and hopefully, in the next few years, we’ll actually be able to move forward.”
Pharmaceutical companies have been among the earliest healthcare companies to partner with digital health startups and pilot digital health tools. While MobiHealthNews has tracked very few digital health product launches from pharma companies themselves, they have still been very active. In the section to follow MobiHealthNews has put together a comprehensive retrospective of our pharma-related coverage from the past four years.
2010 Q1
 In the beginning of 2010, it became clear that pharmaceutical companies’ roles are shifting as they begin to become a more active part of the care
In the beginning of 2010, it became clear that pharmaceutical companies’ roles are shifting as they begin to become a more active part of the care
equation. PricewaterhouseCooper pointed out that “by supporting care delivery innovations, pharmaceutical companies will rebuild trust among consumers and be seen as a strong partner in delivering effective healthcare. The need for consumer engagement at all points of care will open up opportunities for pharma to partner with providers and insurers to increase the value of health spend and effectively manage at-risk populations.” In other words, consumers will become fed up with waiting for care, so PwC predicts they will turn to every other available option.
While the deal between Novartis and Proteus Digital Health was not originally announced during the first quarter of 2010, the details surrounding the two companies agreement became clearer:
Pharmaceutical giant Novartis announced that it would invest $24 million in upfront cash and equity as part of an exclusive worldwide agreement to license Proteus Digital Health's sensing technology for organ transplantation. Proteus system includes a wireless band-aid like sensor and technology that embeds into a pill. The “intelligent pills” run on an electric charge generated by the patient’s stomach acid. The charge is detected through the patient’s body by a sensing patch on the patient’s skin. The patch records the time and date that the pill is digested and also measures certain vitals, which the company calls the Sentinels of Wellness, including heart rate, activity and respiratory patterns. The information is then sent to the patient’s mobile phone and then onto a portal for caregivers to review and analyze. As part of the deal, Novartis also has “certain” options rights related to oncology, cardiovascular, and clinical development. To date, Proteus has focused on cardiovascular disease, tuberculosis and psychiatric disorders applications for its technology.
The venture arm of Novartis also participated in a round of funding for Bedford, Massachusetts-based MicroCHIPS, which is developing an implantable medical device that will deliver drugs inside the body. This $16.5 million third round of funding brings the company’s total funding to just north of $70 million.
 While Novartis appeared to be the pharmaceutical company most financially committed to the wireless health industry during Q1 2010, the wireless health start-up that generated the most news was undoubtedly Vitality, the maker of GlowCaps.
While Novartis appeared to be the pharmaceutical company most financially committed to the wireless health industry during Q1 2010, the wireless health start-up that generated the most news was undoubtedly Vitality, the maker of GlowCaps.
St. Louis-based pharmacy benefit manager Express Scripts announced its plans to launch a national pilot for Vitality’s GlowCap pill reminder device. A GlowCap is a high-tech top for a standard pill bottle. The GlowCap is equipped with a wireless transmitter that plugs into the wall and looks like a nightlight. When it’s time for a dose of medicine, the GlowCap will emit a pulsing orange light. If the patient misses the dose, an hour later the GlowCap will beep every five minutes. If that doesn’t get the pillbox opened, the device can send automated phone calls or text message reminders to patients who fail to take their pills. Finally, the device can send emails to a family member or doctor with reports on the patient’s overall compliance.
Vitality, the developer of GlowCaps, also announced that “four of the top pharmaceuticals companies have committed to distribute their medications for hypertension, transplants and diabetes in GlowCaps.” Those partnerships have continued to remain undisclosed.
Pfizer inked a deal with former Google Health head Adam Bosworth’s new startup Keas. Keas operates an online care plans store, similar to an app store, that enables users to pick and choose the type(s) of care programs they want to follow and then coaches them to stay adherent to the plan. While Pfizer did not create specific plans for Keas, the company seems to have made an investment in Keas and announced plans to work with the startup on crafting the site’s offerings.
2010 Q2
 No discernible trend here among the pharmaceutical companies and pharmacy groups, but there were a few notable mobile health launches during the three-month period:
No discernible trend here among the pharmaceutical companies and pharmacy groups, but there were a few notable mobile health launches during the three-month period:
US-based drugstore chain Walgreens launched a new text message (SMS) powered prescription alerts service for its customers. Prescription Text Alerts notify customers when their prescriptions are ready. Walgreens also re-launched its iPhone app as well as its mobile site, m.walgreens.com.
Epocrates inked a deal with Pfizer to add a new feature to its iPhone application: Healthcare providers can now access the Pfizer Medical Information Group and obtain scientific answers to their product questions or to report an adverse event. “Contact Pfizer” is now a feature available in the Epocrates drug profiles of approximately 40 Pfizer products, according to Epocrates. Healthcare providers will soon have the option to email Pfizer Medical Information for select Pfizer products, the companies said in a press release.
Rose Crane, CEO of Epocrates: “It is at this ‘moment of truth’ physicians may have a question only the manufacturer can answer. We are the only company in a position to facilitate that direct link, and significantly advance communication between a manufacturer such as Pfizer and our strong network of HCPs.”
Bayer HealthCare Pharmaceuticals launched a mobile app, called FactorTrack, which Bayer bills as the first customizable mobile application for people with hemophilia A. FactorTrack is a free, personal and interactive mobile application that helps make it easier to track and record hemophilia factor VIII infusions.
2010 Q3
Big pharma and retail pharmacies made a couple of important (but very different) announcements during the third quarter.
 CVS Caremark launched a free iPhone app that allowed its pharmacy benefits manager members to look up drug information, refill prescriptions, check prescription status, view prescription history, check drug costs and more. The app even enables users to request a new prescription from their care providers. Surprisingly, CVS Caremark only launched the app for the iPhone platform, which makes it unavailable for the majority of its customers.
CVS Caremark launched a free iPhone app that allowed its pharmacy benefits manager members to look up drug information, refill prescriptions, check prescription status, view prescription history, check drug costs and more. The app even enables users to request a new prescription from their care providers. Surprisingly, CVS Caremark only launched the app for the iPhone platform, which makes it unavailable for the majority of its customers.
French pharmaceutical company Sanofi Aventis announced that it was working with medical device maker Agamatrix to create a blood glucose meter plug-in for Apple’s iPhone called iBGStar.vAccording to the companies, the meter could be the first medical device plugin to connect to Apple’s iPhone. While Johnson & Johnson company Lifescan popularized the idea of connecting blood glucose meters to the iPhone and other smartphone in 2009 during an Apple event, Lifescan has yet to launch such a device. iBGStar, for that matter, is also not yet commercially available in the US, it still needs to receive the greenlight from the FDA. A representative for the companies said that the product should be on the market in January or February of 2011.
The forthcoming iPhone BGM plug-in will interact with a not yet Apple-approved iBGStar Diabetes Manager App that will help users track blood glucose, carbs intake and insulin dose. Agamatrix already offers such an app for iPhone users under its Wavesense brand, however, that application does not pair with a blood glucose meter.
Manhattan Research analyst Monique Levy believes that pharma should be focusing on marketing to healthcare professionals not consumers via the mobile marketing channel:
“The opportunity for healthcare marketers to leverage mobile marketing for patients in this time frame is comparatively less strong. As with PC-based access, patients predominantly use mobile devices to look for health information today; relatively few have or are interested in using tools or services to help them manage their care or benefits,” Levy said. “What’s more, adoption and interest in health mobile activities skew towards younger age groups, which typically have a lower incidence of chronic conditions.”
2010 Q4
 Apart from a number of smartphone app launches -- including AstraZeneca UK's first app, an education resource for clinicians on epidermal growth factor receptor (EGFR) gene-testing in lung cancer -- the biggest news to come out of the pharma stakeholder group in mobile health was Novartis' announcement that it would seek to attain regulatory approval for Proteus Digital Health's microchipped pills within the next 18 months. Proteus' technology embeds intelligence into the pills themselves and tracks medication adherence by time-stamping the patient's ingestion of medications. Novartis global head of development Trevor Mundel told attendees at the recent Reuters Health Summit in New York that it plans to submit the smart pill system for regulatory approval in Europe sometime in 2012.
Apart from a number of smartphone app launches -- including AstraZeneca UK's first app, an education resource for clinicians on epidermal growth factor receptor (EGFR) gene-testing in lung cancer -- the biggest news to come out of the pharma stakeholder group in mobile health was Novartis' announcement that it would seek to attain regulatory approval for Proteus Digital Health's microchipped pills within the next 18 months. Proteus' technology embeds intelligence into the pills themselves and tracks medication adherence by time-stamping the patient's ingestion of medications. Novartis global head of development Trevor Mundel told attendees at the recent Reuters Health Summit in New York that it plans to submit the smart pill system for regulatory approval in Europe sometime in 2012.
Walgreens announced a small but important deal with Epocrates during the quarter: Physicians who use Epocrates mobile applications will be able to find lower cost medications for their patients thanks to an integration with the Walgreens Prescription Savings Club (PSC) formulary list. More than 2 million patients are enrolled in Walgreens PSC discount club, which covers commonly prescribed medications as well as lifestyle drugs for weight management, smoking cessation and family planning. The partnership will likely lead to some physicians recommending that their patients join Walgreens PSC to save on medications, but it will also help care providers help patients stay adherent to medication regimens.
2011 Q1
Ernst & Young issued a report during the first quarter that found that pharmaceutical companies led by Merck and Novartis have increased their investments in mobile phone apps and educational websites by 78 percent year over year. These apps and sites generally aim to encourage patients to take their medications, eat well and exercise more often, according to the report. Pharma companies launched a total of 97 projects that made use of information technologies to improve patient health last year.
Those 97 projects amount to an impressive figure, especially since pharma launched 127 such projects during the previous four years combined.
Interestingly, about 41 percent of these projects were smartphone applications, according to the report. That marks an 11 percent increase in mobile health launches for pharma since 2006.
 Meanwhile, pharmaceutical blog InPharm noticed that Pfizer, Johnson & Johnson and Merck had withdrawn nine smartphone apps from appstores. Each of the companies continue to support otherapps they have developed for the iPhone platform, however. It’s to be expected, of course, that not all apps will stick around – especially those developed to market a certain brand of drug that is no longer a priority.
Meanwhile, pharmaceutical blog InPharm noticed that Pfizer, Johnson & Johnson and Merck had withdrawn nine smartphone apps from appstores. Each of the companies continue to support otherapps they have developed for the iPhone platform, however. It’s to be expected, of course, that not all apps will stick around – especially those developed to market a certain brand of drug that is no longer a priority.
Apps aside – one device company that had been attracting pharma companies became one of the first high profile acquisitions in mobile health. Pharma entrepreneur and billionaire Dr Patrick Soon-Shiong acquired Vitality, the company that developed GlowCaps. Vitality CEO David Rose stated in the company press release that while the financial terms of the acquisition have not been disclosed, Vitality’s investors achieved a ten-fold return on their investments. In 2009 InformationWeek reported that the company had raised about $4 million, including investments from Dr. Soon-Shiong. Rumor has it the company fetched a price tag of around $25 million.
After just four months of launching “Refill by Scan,” Walgreens found that users of its smartphone application embraced the feature that enables them to use the camera on their phone to scan the barcode printed on a prescription label to order a refill. Walgreens said half of all refill orders originating from a mobile device are now from Refill by Scan. The feature has been available to users of Walgreens’ iPhone and Android apps since November 2010. Walgreens also announced that more than 1 million people have subscribed to its prescription text alert service, which informs customers when a prescription is ready for pickup. That service was launched in March 2010.
Finally, the first quarter also saw the initial public offering (IPO) of Epocrates, a physician reference application company that makes a good portion of its money from pharmaceutical sponsorship and partnerships. Epocrates raised more than $86 million with its IPO and began trading well over its initial share prices in the weeks to follow.
At the time of its IPO, Epocrates said some 45 percent of physicians in the US use its app, which helps them look up drug data on Apple, BlackBerry and Android devices, phones and some tablets. Epocrates makes money through deals with pharma companies that send “clinical alerts” through its platform. It also has a premium version of its app.
2011 Q2
Pfizer made big news during the second quarter by announcing the first FDA-approved clinical drug trial involving all-electronic homebased reporting. Pfizer tapped Exco InTouch to leverage the vendor’s eDiary technology for the mobile-enabled Participatory Patient-Centered (PPC) clinical trial. Trial participants will receive medication via the mail and use the mobile application to participate in the study. The drug being “tested,” Detrol, is intended for use by patients with overactive bladders and has already been FDA-cleared. The study is officially called the Research on Electronic Monitoring of OAB Treatment Experience (REMOTE) Phase 4.
The real purpose of the study, of course, is to compare the mobile reporting of trial data to traditional methods of drug testing. The drug being used had previously been tested against a placebo during a four month 600 patient trial in 2007.
 The trial is also expected to bring about dramatic cost savings largely because trial participants will not be required to go to a testing facility at any point during the study. Participants will not be required to live near a test site either. Pfizer plans to recruit them via online ads. The company also plans to have participants to track symptoms via the app, conduct blood tests at home, and fill out periodic assessments online.
The trial is also expected to bring about dramatic cost savings largely because trial participants will not be required to go to a testing facility at any point during the study. Participants will not be required to live near a test site either. Pfizer plans to recruit them via online ads. The company also plans to have participants to track symptoms via the app, conduct blood tests at home, and fill out periodic assessments online.
Another large pharma company, Medco Health Solutions, made news during the quarter: The pharma company inked a deal with Verizon Wireless to offer a mobile medication app. The app, which will be available to Android and BlackBerry users on the Verizon Wireless network, aims to provide users with information about lowest cost prescription options and potentially harmful drug interactions based on the user’s prescription history. The free app, called Medco Pharmacy, is available from the mobile operator’s V CAST store. The Medco has three core offerings: My Rx Choices, My Medicine Cabinet, and Prescription ID Card. My Rx Choices provides users with personalized out-of-pocket costs for any prescription medication along with lower cost alternative medications based on the member’s pharmacy plan. My Medicine Cabinet lets users view the medications they are currently taking and also can remind users to refill them. The Prescription ID Card offers up access to the user’s ID card information right from the app.
2011 Q3
While Pfizer inspired headlines last quarter by announcing the first FDA-approved remote clinical study supported by mobile phone data selection, the third quarter of 2011 brought with it very little mHealth activity from pharma.
Pfizer did announce the name of the vendor it is working with for its REMOTE trial: Exco InTouch, which ended up receiving nearly $5 million in growth equity from SEP at the end of the quarter. Pfizer and Exco began due diligence for the trial by piloting a text messaging program to ensure that Phase I volunteers stick to their medical appointments. Patients who agree to participate receive text messages from Pfizer via Exco InTouch, which is a UK-based mobile consultancy that says it has provided texting services in more than 20 clinical trials globally. While Pfizer awaits the results, Exco InTouch says its programs reduce follow-up rates (for appointments) by 20 percent and boost recruitment responses by 500 percent.
While it’s not news, during the third quarter HealthEd’s Director of Strategic Services, Jeff Greene outlined a handful of ways that pharmaceutical companies could be making use of mobile apps and other technologies. The three main buckets Greene placed the pharma mHealth opportunity into were: Branding, medication adherence, and clinical trials.
 Greene wrote that pharma companies were experimenting with SMS short codes and outdoor advertisements (billboards, and a jumbo-tron in Time Square, etc.) to drive customers to their mobile sites. The customer is incentivized to interact with the program because they are given a voucher or coupon that can be redeemed at their pharmacy. Those potential customers without smartphones can just use SMS to text their email address to the server to receive the coupon. Greene said that medication adherence supported by mobile is one of the “most exciting” areas for pharma. The “humble” refill reminder is one strategy. Finally, using mobile for clinical trial management is a third opportunity. Mobile can help recruit patients, manage the study, and lead to better patient-reporting.
Greene wrote that pharma companies were experimenting with SMS short codes and outdoor advertisements (billboards, and a jumbo-tron in Time Square, etc.) to drive customers to their mobile sites. The customer is incentivized to interact with the program because they are given a voucher or coupon that can be redeemed at their pharmacy. Those potential customers without smartphones can just use SMS to text their email address to the server to receive the coupon. Greene said that medication adherence supported by mobile is one of the “most exciting” areas for pharma. The “humble” refill reminder is one strategy. Finally, using mobile for clinical trial management is a third opportunity. Mobile can help recruit patients, manage the study, and lead to better patient-reporting.
2011 Q4
Marty DeAngelo, vice president and director of interaction design at Digitas Health, believes that pharmaceutical companies are failing to capitalize on the rise of the mobile channel as a way to reach consumers and healthcare professionals. In a column over at MediaPost, DeAngelo claims that of the top 25 drug brands in 2010, only three had mobile websites as of December 2011.
“Plavix has a brand site for consumers, Lipitor Savings provides information to consumers about their savings program, and only Nexium has a mobile site specifically aimed at HCPs,” DeAngelo wrote. “In fact, based on recent research I’ve conducted, there are only a handful of mobile websites in all of the pharma space – 15 at my last count.”
DeAngelo chalks up pharma’s inaction to three major reasons: a lack of understanding of mobile use cases, a misunderstanding of how to convey information in a mobile context, and regulatory discomfort.
 One sign of a potential rise in action from pharma companies looking to leverage the growing adoption of mobile services by consumers and providers alike was the launch of the Tomorrow Networks mobile advertising network. The ad network officially launched in the fourth quarter to serve ads specifically targeted to healthcare providers and health-conscious consumers. Physicians Interactive Holdings, which offers the popular Skyscape app, and stealthy Remedy Systems partnered to launch the mobile ad network, called Tomorrow Networks. The network launched with some 54 different smartphone medical applications having already signed on. Tomorrow Networks is intended to be a revenue channel for those medical apps that haven’t monetized yet or those looking for an additional incremental revenue stream. According to the launch press release, Tomorrow Networks already claims to have an app user base of 275,000 healthcare professionals.
One sign of a potential rise in action from pharma companies looking to leverage the growing adoption of mobile services by consumers and providers alike was the launch of the Tomorrow Networks mobile advertising network. The ad network officially launched in the fourth quarter to serve ads specifically targeted to healthcare providers and health-conscious consumers. Physicians Interactive Holdings, which offers the popular Skyscape app, and stealthy Remedy Systems partnered to launch the mobile ad network, called Tomorrow Networks. The network launched with some 54 different smartphone medical applications having already signed on. Tomorrow Networks is intended to be a revenue channel for those medical apps that haven’t monetized yet or those looking for an additional incremental revenue stream. According to the launch press release, Tomorrow Networks already claims to have an app user base of 275,000 healthcare professionals.
Merck Canada announced a deal with Mihealth to promote its PHR offering to Canadian doctors. According to Mihealth, which has an exclusive license to Diversinet’s MobiSecure platform in Canada, its PHR data is validated by a physician once a year to ensure accuracy of the health information. Mihealth’s PHR is available for iOS, Android, BlackBerry and Windows smartphones. The software helps patients track medications, allergies, and chronic disorders information, but it has plans to add lab tests information soon. The Mihealth service is free for physicians while patients pay from $59 a year per person and up to $224 for a family of four. Mihealth pays physicians a small fee for their yearly validation.
On the retail pharmacy side, Walgreens announced the launch of an SMS reminder system for prescription refills. The free service reminds patients about medications due for a refill and makes it easy for them to replenish their meds: Patients need only reply “refill” to the reminder text.
London-based Exco InTouch, which is the vendor powering Pfizer’s high-profile mobile-enable clinical raised about $4.67 million from Scottish Equity Partners (SEP). Exco plans to use the funds to expand and take advantage of the “significant growth opportunities” in the emerging mobile patient communication market, the company’s press release stated.
PositiveID announced that its iGlucose diabetes management system will be used in a study sponsored by Sanofi and the American Medical Directors Association Foundation (AMDA). PositiveID’s iglucose system pairs with compatible blood glucose meters to wirelessly submit readings to a diabetes management portal, which can then by viewed via electronic logbooks and aggregated into trend reports. Readings can be shared with caregivers and healthcare professionals via text message, email, or fax.
2012 Q1
 Proteus Digital Health Raisin system
Proteus Digital Health Raisin system
During the first quarter research analyst firm Cutting Edge Information (CEI) published a report that suggested pharma companies look to clinical trials and other pre-launch activities as the ripest opportunities for leveraging mobile health apps. “With usage retention rates likely to continue hovering in the single digits, the industry may soon conclude the market is saturated with mobile health apps,” CEI wrote. “Despite the low cost of development, a reasonable [ROI] is much more difficult than first envisioned.” CEI suggests two areas where pharma should focus: Streamlining trial data collection and analysis, and connecting potential trial patients to investigators. Those were opportunities for pharma companies to “differentiate themselves from the pack,” according to CEI.
Proteus Digital Health inked a deal with Lloyd’s Pharmacies in the UK to offer Helius, an intelligent medicine service, to pharmacy customers. Lloyds will offer the Helius system as part of a personalized, medication adherence pack to its customers, which its pharmacists assemble for each individual customer. The Helius packs will include components of the Helius system as well as blister packs of the customer’s drug regimen. For example, if a person takes five medications each morning, one blister pack would include all five of those medications, but it will also include a Helius tablet.
The Helius tablet is a sensor-enabled pill that communicates to a peel-and-stick sensor patch worn on the patient’s body. When the patient ingests the tablet and it breaks down, it sends a signal to the patch to indicate the dose had been taken. The patch communicates this information to an app on the user’s mobile phone. The patch doesn’t only record when a pill is ingested, it also tracks sleep patterns and records physical activity levels.
Another notable mobile health launch in the UK: Pharmaceutical giant GlaxoSmithKline (GSK) offered up a free asthma management app, called MyAsthma, for iPhone and Android users. The app’s core offering is an Asthma Control Test (ACT), which is a simple 30-second test that provides users with an index score for how well they are managing their asthma overall. UK-based Replay Digital developed the app for GSK. Dr. Mike Thomas, Senior Research Fellow with the University of Aberdeen and Chief Medical Advisor, Asthma UK, helped develop the app along with Professor Rob Horne, a behavioural psychologist at the University of London.
 The MyAsthma app keeps users posted on local information about asthma triggers, including pollen count, pollution, and weather. It also helps users track symptoms and their ACT scores over time to see trends that they can share with care providers. GSK has also developed an algorithm based on this data that generates a selection of messages to the user based on their ACT scores and other data.
The MyAsthma app keeps users posted on local information about asthma triggers, including pollen count, pollution, and weather. It also helps users track symptoms and their ACT scores over time to see trends that they can share with care providers. GSK has also developed an algorithm based on this data that generates a selection of messages to the user based on their ACT scores and other data.
2012 Q2
In recent years Apple stores began selling fitness devices other than those offered by long time partner Nike+. The company added iHealth’s iOS Blood Pressure Monitor dock as the first FDA cleared device on its store shelves. Soon followed Withings’ WiFi-enabled scale and more. This week Cupertino’s retail stores have begun selling Sanofi’s iBGStar device, the first FDA cleared iPhone-enabled blood glucose meter. Apple’s online store and Walgreens.com are also selling.
Apple is offering the iBGStar for about $100 while Walgreens has priced it at about $75. An iPhone or iPod touch is not included, of course, and while the meter works without the device, much of its value would be lost without one. Since Apple will not be selling testing strips for the device separately, the iBGStar sold at Apple stores include 50 strips, which explains the higher price.
While Walgreens has an exclusive arrangement with Sanofi to sell the device and strips, Sanofi said that any pharmacist can order the device for a patient through McKesson.
Sanofi wasn’t the only pharma company to make mobile health announcements during the quarter, Pfizer announced a partnership with the magazine EatingWell to co-launch a mobile app aimed at patients who take Lipitor. While there are a few mobile apps in the market specifically focused on one particular pharmaceutical product, it’s a safe bet that none were launched eight days before multiple generic versions of that drug hit pharmacists’ shelves.
“This is Pfizer’s first foray into a prescription drug-affiliated app,” health economist Jane Sarasohn-Kahn wrote at the time. “The free mHealth app, Recipes 2 Go, was launched as part of the Lipitor Smart Living website and is available from the Apple App Store for iPhone, iPod touch, and iPad, and for Android phones and tablets from Google Play. The app includes recipes from EatingWell magazine, a shopping list tool, a timer for cooking, and a recipe search finder. The app also has a copy of the Lipitor $4 co-pay card where patients enrolled in the Pfizer Lipitor for You program can keep their ID and use the digital card for refilling Lipitor prescriptions at the pharmacy.”
Another glucose meter technology developer announced a partnership with a leading pharma company: Glooko, a mobile diabetes management startup, inked a deal with Roche Diabetes Care to extend the Glooko MeterSync to ACCU-CHEK meter users via an infrared data transfer adapter. The Glooko IR Adapter helps iPhone or iPod touch users who would like to more easily download blood glucose readings from their ACCU-CHEK meters to the Glooko Logbook app on their iOS device. The infrared adapter works with three ACCU-CHEK meters: ACCU-CHEK Aviva, ACCU-CHEK Compact Plus, and the ACCU-CHEK Nano SmartView Meter System.
2012 Q3
During the third quarter Proteus Digital Health, formerly known as Proteus Digital Health, became the first company to receive Food and Drug Administration clearance for an ingestible biomedical sensor that monitors medication adherence.
The FDA granted 510(k) premarket approval to the Proteus Ingestible Event Marker (IEM) as a de novo medical device, meaning that there was no similar product on the market, four years after Redwood City, Calif.-based Proteus first sought clearance. The FDA cleared a related wireless sensor patch developed by Proteus in 2010.
The IEM sensor, which can be embedded into a pill, is activated by stomach fluid, then transmits a signal through the body to the skin patch, indicating that a patient has ingested medication. The patch also measures patient vitals and body position, and wirelessly sends all of the data to a Proteus smartphone app.
Shortly before securing its FDA clearance, Proteus announced that it had inked a deal with Japan-based Otsuka Pharmaceutical, which is known for its Abilify drug for schizophrenia and bipolar disorder. The deal will bring technology based on Proteus Digital Health’s Raisin platform to that country.
Over the summer Janssen Healthcare Innovation, an entrepreneurial unit of pharmaceutical heavyweight Johnson & Johnson, began promoting medication adherence with a new secure messaging platform and smartphone app and website.
The Care4Today Mobile Adherence program delivers reminders to take medications as directed, refill prescriptions and visit physicians when appropriate. Users can request reminders for any prescription – whether or not it’s a J&J drug – or for overthe- counter medications and nutritional supplements.
Care4Today Mobile Adherence is available as an iPhone/iPad and Android app, but it also works with any web-enabled phone or PC. Users sign up for free on the website and register the medications they want reminders for. The reminders can be specific or discreet, with coded phrases such as “walk the dog,” Janssen says. Each message asks for a response to indicate that the patient has taken medications. The system keeps a log for users to monitor their own adherence and, if they choose, bring a printout to share with their doctors. For the sake of privacy, Janssen Healthcare Innovation says it does not have access to user identities.
2012 Q4
Although there are rumors of big moves in 2013, pharmaceutical companies laid low at the end of 2012.
 Omniscience Mobile's clinical trial app.
Omniscience Mobile's clinical trial app.
In September, 10 pharmaceutical companies – Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Pfizer, Roche’s Genentech division, and Sanofi – banded together to start a nonprofit called TransCelerate BioPharma, which will focus on improving the efficiency of clinical trials. Although TransCelerate’s initial goals aren’t mobilefocused, they could pave the way for mobile engagement in the future. One goal is to create a shared Internet portal for investigators to communicate with drug companies and another is to adopt a standardized data format for clinical trial recruiting, both of which will make data easier to manage in any format.
Rather than having a different system and a different login username and password for each drug company an investigator works with, the shared portal initiative, spearheaded by Johnson & Johnson’s Janssen Clinical Trials Innovation Unit, would create a single sign-in that would give investigators access to all the drug companies they’re working with. The data, including proprietary information on specific drugs, would still be firewalled and stored separately, but it would be accessed uniformly.
Separately from Transcelerate BioPharma, Janssen’s Clinical Trial Unit is working on a shared online databank of all non-proprietary clinical information. This includes investigator training and capabilities and standard trial practices and procedures – nearly anything that isn’t related to particular drugs. They’re also developing a digitized informed consent form and electronic blister to track medication adherence.
In other news, Pfizer’s Craig Lipset said in November that Pfizer would be relaunching its all-mobile clinical trial, which failed in March 2012, again in 2013. Pfizer’s study, a clinical trial involving an overactive bladder treatment launched in the summer of 2011, was poised to be the first clinical trial that patients could participate in remotely, with even the drugs delivered to the patients by mail. Previously, Pfizer had conducted a traditional trial on the same drug, Detrol LA. The pilot was focused on testing the online method itself and on promoting patient engagement in the research.
 Pfizer announced a year later that they were discontinuing the trial, however, citing a lack of participation. Lipset said at the time that though social media channels from Facebook to Craigslist were successful in getting people to the website, the number of those visitors who actually signed up for the clinical trial was too low, possibly because patients didn’t trust online sources enough to deliver the necessary personal information. Lipset said that the failure might have been due to trying too many new innovations at once. Pfizer is going to try that trial again in 2013, tweaking its approach based on lessons from the initial experiment.
Pfizer announced a year later that they were discontinuing the trial, however, citing a lack of participation. Lipset said at the time that though social media channels from Facebook to Craigslist were successful in getting people to the website, the number of those visitors who actually signed up for the clinical trial was too low, possibly because patients didn’t trust online sources enough to deliver the necessary personal information. Lipset said that the failure might have been due to trying too many new innovations at once. Pfizer is going to try that trial again in 2013, tweaking its approach based on lessons from the initial experiment.
In December, the FDA approved its first remote monitoring trial, a 12-month Phase 2 trial by biopharma startup Transparency Life Sciences. For the trial multiple sclerosis patients will take the blood pressure drug lisiniprol and wear vital sign monitors provided by AMC Health. The trial will include 180 subjects and those with wearable vital sign monitors will transmit their data to investigators and also conduct secure video conferences with them. The trial is estimated to save $3.5 million over a traditional trial.
MobiHealthNews learned during the quarter that Omniscience Mobile, a mobile clinical trial startup, has been working with pharmaceutical companies including Merck and Pfizer. Omniscience’s clinical trial work is currently text-massage based and focused on recruiting, but the company is releasing an app in 2013 that will address retention, informed consent, and provide an e-diary for more accurate self-reporting.
2013 Q1
Both pharma companies and retail pharmacies made big moves in the first quarter of 2013. When the year first kicked off, MobiHealthNews heard rumblings from multiple sources that this year would be a watershed one for pharma-driven mobile health services.
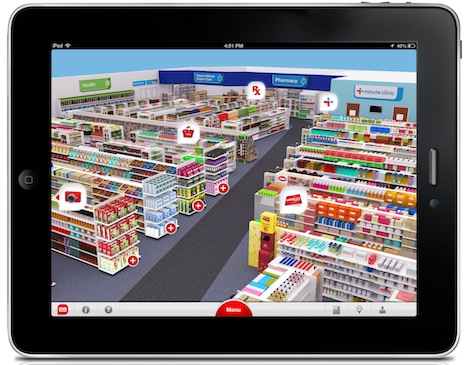
CVS Caremark made a bit splash with its new iPad app.
The CVS iPad app presents the user with a 3D virtual pharmacy, where each section of the store corresponds to a functionality for the app, many of which were already present on CVS’s iPhone app. In the Pharmacy, users can scan barcodes to refill prescriptions and manage their family’s prescriptions. In the MinuteClinic, users can search for nearby clinics, check whether their insurance is accepted, and see a list of services offered.
There are also non-health related sections: ExtraCare, which manages coupons and reward programs, and functions for the store itself, online shopping, and photo development.
 Nant Health-owned Vitality made their GlowCap pill container caps available for direct-to-consumer purchase from CVS during the quarter, too. Vitality has also been quietly developing a new product, the Vitality GlowPack, a customizable pouch which provides similar functionality to the GlowCap. The advantage to the GlowPack is that it can be used it for medications larger than pills.
Nant Health-owned Vitality made their GlowCap pill container caps available for direct-to-consumer purchase from CVS during the quarter, too. Vitality has also been quietly developing a new product, the Vitality GlowPack, a customizable pouch which provides similar functionality to the GlowCap. The advantage to the GlowPack is that it can be used it for medications larger than pills.
CVS Caremark and Vitality GlowCaps are working together on a new randomized control trial using GlowCaps to test the effectiveness of sweepstakes to improve medication adherence.
The study, led by the University of Pennsylvania, along with Carnegie Mellon University and Rutgers University, will include 800 high-cholesterol participants in four groups. Each group, including the control group, will use Vitality GlowCaps to remind them to take cholesterol-lowering drugs, or statins, but each experiment group will also be enrolled in a different kind of sweepstakes. For one group, participants will have the chance to win money each time they remember to take their medication. One group will be eligible to win only if they take their medication prior to being reminded. A third group will accumulate money in an account each time they successfully take their medication on time, but the account will only pay out if they reach a certain adherence level. Recruitment for the study starts this month and data collection for the study concludes in August 2016.
Meanwhile, Walgreens introduced an application programming interface (API) so software developers can build the company’s prescription scanning and refilling technology into their own apps. The functionality already is in drug information app PocketPharmacist and newly launched personal health record Healthspek, according to Deerfield, Ill.-based Walgreens.
 The basic Walgreens app is widely used for finding retail locations, refilling prescriptions by scanning bar codes, setting personal medication reminders, looking up medication information, ordering photographic services and making appointments at in-store Take Care Health System clinics. Walgreens noted that more than 40 percent of its online refill requests now come from its app.
The basic Walgreens app is widely used for finding retail locations, refilling prescriptions by scanning bar codes, setting personal medication reminders, looking up medication information, ordering photographic services and making appointments at in-store Take Care Health System clinics. Walgreens noted that more than 40 percent of its online refill requests now come from its app.
Novartis Oncology, a division of the Switzerland-based pharmaceutical company, has added two new free Apple and Android apps to its catalog: My NET Manage and Clinical Trial Seek. Interestingly, one of their most high-profile apps, VaxTrak, has now disappeared from the Apple and Google Play stores.
My NET Manager is an app for patients with neuroendocrine tumors (or NET) to help them manage and learn about their condition. It provides tools for users to track their symptoms, set reminders for treatments, doctor visits, and medication refills, and review and track test results. The app also stores contact information for a patients’ healthcare team and enables the patient to share information with their team. Finally, the app can help NET patients track insurance claims.
The other new app, Clinical Trial Seek, is sourced from the National Institutes of Health’s clinicaltrials.gov database. The app is designed to help doctors and patients find cancer clinical trials in their area, and to help patients learn more about clinical trials. Users can search trials by location, disease type, treatment, phase of trial, trial sponsor, eligibility requirements or keywords, and can save and email their results.
In the UK, pharmaceutical company AstraZeneca announced a pilot with Exco InTouch, to help patients with chronic obstructive pulmonary disease (COPD) with mobile and online tools.
The pilot will be administered via several National Health Service Clinical Commissioning Groups and funded by AstraZeneca, with Exco InTouch providing the technology and outreach to providers. The service will provide patients with an interactive app, through which Exco InTouch, AstraZeneca, and care providers will send patients educational materials and patients will track symptoms and treatments by filling out surveys. Patients will also use a Bluetooth-enabled inhaler offered by AstraZeneca that will automatically report data.
Mobile software developer Mobile Commons, pharmaceutical company Sanofi US and the Prostate Cancer Foundation announced the creation of Prost8Care, an SMS system to help prostate cancer patients and their families understand treatment processes.
Patients will enroll via their doctors. The program will send printed informational and enrollment material to physician offices. Once patients are signed up, they should start receiving text messages, with tips on eating properly and coping with chemotherapy side effects, timed to each man’s treatment cycle. Prost8Care is a one-way messaging program, so participants will not be asked to respond to the texts. There is no cost other than carrier messaging charges.
 The Merck Global Health Innovation Fund, a unit of pharmaceutical giant Merck, led a $20 million investment round in PatientSafe Solutions. Other investors included Camden Partners, TPG Capital and Psilos Group. As a result of the investment, San Diego-based PatientSafe has added Max Kahn, a Merck GHI principal, to its board.
The Merck Global Health Innovation Fund, a unit of pharmaceutical giant Merck, led a $20 million investment round in PatientSafe Solutions. Other investors included Camden Partners, TPG Capital and Psilos Group. As a result of the investment, San Diego-based PatientSafe has added Max Kahn, a Merck GHI principal, to its board.
PatientSafe, formerly known as IntelliDot, will use the money to ramp up marketing of its PatientTouch platform, a point-of-care suite that runs on iPod Touch devices enclosed in waterproof cases. The company also intends to add features aimed at helping hospitals meet “meaningful use” standards for electronic health records, increasing efficiency and preparing for the era of accountable care.
The PatientTouch package currently includes a barcode reader for patient identification and various communications technology to encourage collaboration. An iPhone version of the system supports internet telephony.
2013 Q2
Patient disease community platform PatientsLikeMe has been retooling itself into an open research platform this quarter, and teaming up with several pharma companies in the process. Sanofi inked a deal with PatientsLikeMe to make use of their network to recruit clinical trial participants. This was a follow-up to a similar arrangement with Merck made in 2012. Then in June, at roughly the same time as a $7 million funding raise, the company teamed up with clinical consulting firm InVentiv health to help InVentiv’s pharmaceutical clients with clinical trial recruitment.
PatientsLikeMe isn’t the only digital health startup looking to provide patient data to pharma companies. Treato, an Israeli company that scours thousands of social media sites to glean patient insights about pharmaceuticals and treatments, raised $14.5 million.
 Treato Pharma
Treato Pharma
Treato, of Yehud, Israel, will use the money to set up a US office in New York and to expand its product offering. The company currently has one stateside sales representative. The new investment also will help the company build out its product, perhaps around therapeutic areas rather than just specific drugs, and to expand its reach globally.
Launched about a year and a half ago, Treato has developed an algorithm to search across social media sites large and small, from Facebook on down to local online communities for patients with specific medical conditions, to ascertain what people are saying about medications. Treato looks for patterns that could signify adverse reactions to drugs, off-label uses, reasons why people switch from one medication to another and other bits of intelligence.
The algorithm matches patient discussions to clinically validated information sources to help people understand how drugs actually work and serve as an early warning to many constituencies about potentially harmful side effects. Treato makes its money from a professional version, Treato Pharma, which has been live since October, providing in-depth and specialized metrics – such as what people eat when taking certain medications – for pharmaceutical marketing and safety teams.
Other digital health startups are catching the eyes of pharma companies in the area of medication adherence. Proteus Digital Health, the developers of smart pills that track medication adherence, raised $45 million from investors including pharmaceutical companies Novartis and Otsuka.
The round was led by Oracle, who Proteus is also partnering with. Oracle and Proteus will work together to help investigators in clinical trials to better understand and measure medication ingestion, dose timing, and associated physiologic responses from patients. Proteus’ ingestible sensor technology will be integrated into Oracle’s clinical trial products, including InForm, Data Hub, and its Siebel clinical trial management system.
 Another startup, Mango Health, a Rock Health startup focused on using gamification principles to tackle medication adherence, launched its titular app after months of beta testing, a 16-week pilot, and not a small amount of hype. The company has raised $3 million so far. With Mango’s app, users enter their medications or supplements, timing, and doses. Like many existing adherence apps, Mango can remind patients when its time to take their medication. It also automatically aler ts them to potentially dangerous interactions between medications, or with food and drink. The app also includes a personal health journal.
Another startup, Mango Health, a Rock Health startup focused on using gamification principles to tackle medication adherence, launched its titular app after months of beta testing, a 16-week pilot, and not a small amount of hype. The company has raised $3 million so far. With Mango’s app, users enter their medications or supplements, timing, and doses. Like many existing adherence apps, Mango can remind patients when its time to take their medication. It also automatically aler ts them to potentially dangerous interactions between medications, or with food and drink. The app also includes a personal health journal.
While many medication adherence technologies target older people with their products, Mango’s target demographic is 35 to 55-year-olds who were recently diagnosed with a chronic disease, although the pilot included users as young as 24 and as old as in their late 70s.
The Merck Global Health Innovation Fund, the investment wing of Merck Pharmaceuticals, has made several moves in the digital health space this quarter.
Merck GHI, along with New York-Presbyterian Hospital, will be an initial partner for Princeton, New Jersey-based TigerLabs’ new Innovation Track. The Innovation Track startups will receive all the same benefits as startups in the regular TigerLabs Health accelerator. In addition, they’ll have regular contact (up to one day a week) with one of the partner companies, both of which will be heavily involved in selecting their partner startup. Merck GHI is looking especially for startups focused on data analytics (in a way that is applicable to the pharmaceutical industr y) and New York- Presbyterian is after startups who can help make their internal hospital processes more efficient — anything from marketing to billing.
Merck GHI also headed up a $15 million funding round for data analytics startup Medivo. Medivo’s business model revolves around giving patients easier access to their lab results and giving physicians access to an aggregated dashboard of patient labs and other data, as well as giving patients tools to monitor chronic conditions. Medivo CEO and co-founder Sundeep Bahn told MobiHealthNews that the company has seen rampant growth over the last few years, doubling its growth each year.
2013 Q3
Retail pharmacy heavyweight CVS Caremark added a drug interaction checker to its CVS Mobile app that cautions consumers when an over-the-counter medication might interact with other drugs they are taking. The Woonsocket, RIbased company says it is the first drugstore chain to include such a feature in a mobile app.
 With the new interaction checker, users can scan the barcodes on OTC medication packages with their phones or enter the name of the drug or an active ingredient to bring up a list of potential interactions. For those with a myCVS online account, the app automatically checks the OTC drug against the patient’s pharmacy history, which can be impor ted directly from CVS or manually populated to include other OTC medications and prescriptions filled through other pharmacies.
With the new interaction checker, users can scan the barcodes on OTC medication packages with their phones or enter the name of the drug or an active ingredient to bring up a list of potential interactions. For those with a myCVS online account, the app automatically checks the OTC drug against the patient’s pharmacy history, which can be impor ted directly from CVS or manually populated to include other OTC medications and prescriptions filled through other pharmacies.
Janssen Healthcare Innovations (JHI), a subsidiary of Johnson & Johnson, launched the new version of its free Care4Today medication reminder app and platform, Care4Today Mobile Health Manager 2.0. The new app is not just an update to Care4Today, which launched last July, but a completely new app built in-house, whereas the original was built on Diversinet’s MobiSecure technology. Diversinet’s assets were acquired by IMS Health in August.
“We thought it would be better to own the application,” Dave Tripi, Partner at JHI, told MobiHealthNews. “We didn’t find a vendor that could meet the requirements.”
Software developer Ringful Health, developers of the first mobile health app to integrate with the now-defunct Google Health, has teamed up with Consumer Reports to create an app to help patients choose the most effective, and of ten cheapest, prescription drug for their conditions.
The app, called Best Drugs for Less, lists the best drugs based on “comparative medical research into ef fectiveness, safety, convenience, and side effects,” according to an ar ticle about the app on ConsumerRepor ts.org. However, in most cases, the drug that Consumer Reports has identified as the best is a generic drug, significantly cheaper than the most-of ten prescribed alternatives. Patients can save as much as $275 a month, for instance, by switching from a $296 per month dose of Celebrex to a $21 a month dose of Ibuprofen for osteoarthritis.
WebMD, for the first time the company has bridged its popular consumer-facing app with its also popular healthcare provider facing app, Medscape.
Physicians can now “prescribe” patient education materials to their patients by pushing that content to the patient’s phone. After getting their permission, the physician sends the patient a message with a link to a mobile-enabled website that features the educational material. Once loaded, that page invites visitors to download the WebMD app where they could also view (and save) the material for later viewing. Or, the patient can just view it on the mobile site and save the link for later viewing.
2013 Q4
 Johnson & Johnson subsidiary Janssen Healthcare Innovations overhauled its Care4Today medication adherence app at the end of the third quarter. That app made news again this quarter when it became the first medication adherence app to integrate with Aetna’s CarePass platform.
Johnson & Johnson subsidiary Janssen Healthcare Innovations overhauled its Care4Today medication adherence app at the end of the third quarter. That app made news again this quarter when it became the first medication adherence app to integrate with Aetna’s CarePass platform.
Aetna picked Care4Today partly because its app is not specific to Johnson and Johnson medications, but includes a wide-ranging database of drugs. The Care4Today-CarePass integration will feed weekly and monthly medication adherence summaries into CarePass’s dashboard, where an adherence tracker will be displayed alongside a user’s activity, nutrition, and sleep tracking bars. Janssen’s David Tripi said the iOS version of Care4Today is currently integrated with CarePass, and the Android version will be connected by the end of the year.
Head of CarePass Martha Wofford said Aetna fully intends to branch out to other medication adherence apps once it’s tested the waters with Care4Today, and that future integrations with Care4Today and other companies will be bi-directional; as well as apps feeding into CarePass, CarePass will optionally send Aetna members’ medication information to their adherence apps. That means that, as Wofford told MobiHealthNews last summer, CarePass will soon start to deal in protected patient health information.
In October, MobiHealthNews reported on the Merck Global Health Innovation Fund’s August acquisition of Physicians Interactive, maker of mobile medical reader apps Skyscape and Omnio. Chief Medical Officer and senior VP of product management Gautam Gulati said the new ownership would not necessitate a change in the direction for the company, but instead give them access to a wide range of resources.
“Imagine having at your disposal access to some of the largest clients to be your pilot for anything new that you develop,” he said. “What we basically were given at the table was a suite of large scale companies willing to experiment with us in the future. On top of that, we were given a platform within their portfolio, too. We’ve just gone from a small piece in the marketplace to something that’s going to have a substantial presence, given the fact that we have the ability to collaborate with all these other portfolio companies.”
 One thing those partnerships will enable, according to Gulati, is incorporating more relevant information into the app, including clinical information and “swivel” information for doctors to share with their patients at the point of care.
One thing those partnerships will enable, according to Gulati, is incorporating more relevant information into the app, including clinical information and “swivel” information for doctors to share with their patients at the point of care.
During the quarter Mike Shilling, director of business development at Exco InTouch, shared three examples of mobile-enabled clinical trials that finished up or are still underway. The trials each leveraged mobiles in different ways and helped highlight a few of the strategies for deploying them in support of a trial as well as the various benefits. Shilling highlighted three benefits enabled by mobile technology: Larger, more comprehensive trials; shorter, less expensive trials; and near real-time data analysis of patient reported outcomes.
The first trial Shilling described was a phase three clinical trial that spanned a number of countries and its aim was to demonstrate the efficacy of an influenza vaccine in a pediatrics population. Shilling said this trial used a bring-your-own-device approach and that since it was tracking children, it involved their parents or carers, not the patients themselves as the engaged participant. Shilling also discussed a longterm study focused on hear t patients who had already had one hear t-related episode. This outcome event monitoring study has involved about 13,000 patients across 30 countries and has been running for six years and counting. For this study, Exco InTouch employed a one-way SMS messaging service that aimed to keep the patients involved and engaged in the study. The third and final example Shilling shared was about a study in Argentina. This post-marketing study to assess the medication adherence of patients with diabetes in an emerging market included provisioned smar tphones, a connected medical device, and an app.
MobiHealthNews learned about two other pharma trials this quarter, both involving Boehringer Ingelheim. Smart pill bottle startup AdhereTech is doing a trial with the pharma company, about which few details have been released. And Boehringer’s diabetes trial with Telcare, originally announced last year with the now-defunct Healthrageous as a partner, did continue, with Healthrageous replaced by another coaching app.
 AdhereTech's smart pill bottle.
AdhereTech's smart pill bottle.
Digitas Health released results from a study of 2,000 patients and caregivers, and found that 33 percent of respondents reported that either they or their physician had used a mobile device at the point of care. Particularly relevant for pharma was the finding that patients who used mobile devices in the exam room were 80 percent more likely to switch to a different medication than those that didn’t. They were twice as likely to request a specific medication. Those who used devices only in the waiting room, on the other hand, were only 25 percent more likely to switch than non-device users. Digitas Health has said before that mobile is a missing opportunity for pharmaceutical companies, so this data lends more support to that assertion.
Digitas Health released preliminary results from this study in July. At the time, they reported that 90 percent of patients said they would use an app if their doctor prescribed it, compared to industry data indicating that only 66 percent would fill a prescription for medication from their physician.
2014 Q1
We've always categorized pharma movement in digital health as slow but steady, but this quarter saw indications that the sector is starting to pick up speed. A notable IMS report took pharma to task for its slowness to adopt social media, although it noted that Johnson and Johnson is far outstripping its peers in that category. Recently finalized FDA guidance could also move that needle going forward.
Pfizer backed startup Akili in the development of a mobile game to help diagnose patients with Alzheimer's. In the future, the game could be used in the treatment or detection of ADHD and autism, among other conditions.
The biggest news this quarter came from Merck, which unveiled a range of digital and big data projects its been working on with healthcare stakeholders through its group Merck Medical Information and Innovation (M2i2). M2i2′s partnerships fit into three broad categories: capturing the patient voice, clinician-facing technologies, and innovative uses of data.
One partnership under the M2i2 umbrella was a project with Boston Children's Hospital to turn Twitter and Facebook into a new source for sleep health data — data about how many people in a population suffer from insomnia and what they have in common with each other. Merck Global Health Innovation Fund, the investment wing of the company, made a large investment in WellDoc this quarter as well.
A couple of startups eyed the digital clinical trial market for pharma: Medical micro-journaling startup TapTrak hired Efren Olivares, who has a 22-year pharma background including work with Pfizer, Lilly, Baxter and Dura Pharmaceuticals, and goBalto raised $5 million to build out its clinical trial management software.
[Download this entire article as an eBook (PDF) right here.]















