 Palo Alto, California-based G-Tech Medical, which is developing a wearable, disposable sensor patch for patients with gastrointestinal problems, has received funding from investor Peter Thiel's nonprofit fund, Breakout Labs. The grant was for $350,000. G-Tech is also looking to raise additional money to fund its next phase of development.
Palo Alto, California-based G-Tech Medical, which is developing a wearable, disposable sensor patch for patients with gastrointestinal problems, has received funding from investor Peter Thiel's nonprofit fund, Breakout Labs. The grant was for $350,000. G-Tech is also looking to raise additional money to fund its next phase of development.
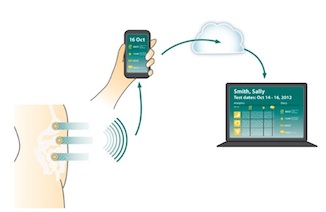
G-Tech's patches measure the electrical activity from the stomach, small intestine and colon -- which the company says is similar to capturing an "EKG for the gut". While the wearables are still in development, they will be thin, conforming, waterproof and comfortable, according to the company. The patches will use Bluetooth LE to send data to the patient's smartphone, which can then send it to a cloud-based portal for physician review. G-Tech is also building an app for physicians that will help them analyze the data, which has never been gathered before, to help them better diagnose functional gastrointestinal disorders. The company also sees an opportunity in testing the effectiveness of therapies.
"This technology that will deliver signals from the GI organs has the potential of demonstrating conclusively that the patient's problems are truly arising from a functional issue and not a manifestation of a serious problem that will require a CT Scan," the company writes on its website. "This would allow the doctor to stop the buck there, reassure the patient and allow immediately the implementation of therapeutic options rather than spending months and years trying to rule out other serious conditions through repeat endoscopies and other invasive examinations."
G-Tech Medical's CEO Steve Axelrod told MobiHealthNews in an interview that the funding from Breakout Labs would help it build the wireless-enabled version of its system, which will be used in a clinical trial of between 50 and 100 patients. The goal is to develop a sensor with at least 24 hours of battery life that can transmit the data to the patient's smartphones and up to the cloud for physician review.
Axelrod said that initial studies have used a wired version of the device in-clinic, which patients have worn while sitting still and watching a three hour movie. While this three hour window is just 1/8 of the day, Axelrod said physicians have already found the data to be useful in diagnosing their otherwise hard to diagnose GI patients. He said that during the 24-hour cycle, the sensor would have four to five opportunities to measure the important electrical signals produced by the digestion process. That's enough data to see early patterns, he said.
G-Tech Medical's roadmap is 38 months long, Axelrod said, and by then he expects to go through two more rounds of funding and have an FDA-cleared system that is ready for manufacture. The first commercial version of the system will have a three-day battery life and would be intended to be worn during that time.


















