 Google-backed 23andMe is finally relaunching its mail-order personal genome service -- just not in the United States. Instead, the company is launching in England, which has a different regulatory framework than the United States.
Google-backed 23andMe is finally relaunching its mail-order personal genome service -- just not in the United States. Instead, the company is launching in England, which has a different regulatory framework than the United States.
"These products are considered to be in the lowest risk category and so there is no requirement for any premarket assessment," a spokesperson for the Medications and Healthcare products Regulatory Agency (MHRA) told MobiHealthNews in an email. "23andMe have included warnings not to change drug therapy or take medically significant action without medical advice."
With its $99 genome tests, 23andMe was one of the rising stars of digital health until about a year ago -- when the FDA sent the company a sternly worded public letter, instructing them to shut down their personal genome service pending regulatory approval. The company complied, but continued to operate its ancestry-based business.
The process of getting back into the FDA's good graces has been a difficult one for the company, and one that other digital health companies are no doubt watching closely. The test itself doesn't require clearance in the US, but the diagnoses and recommendations returned as a result of the test -- telling patients they're at risk for a disease or suggesting that one drug might be more effective for them than another based on their genes -- are a different story. The FDA argues these create medical risks for patients who might alter their therapies based on these results.
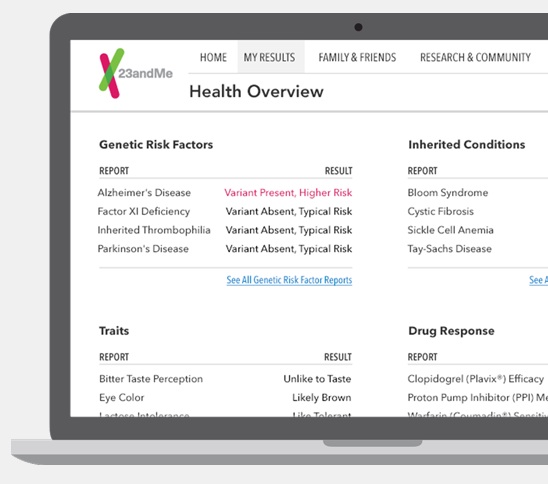
To make matters more complicated, the FDA won't issue blanket clearance for all the diseases 23andMe purports to predict or check for. So in order to be in compliance, the company would have to get each health report cleared. They took a first step back in June, according to the company blog, when they submitted their health report for Bloom Syndrome to the FDA. The test doesn't appear to have been cleared yet.
"Once cleared, it will help 23andMe, and the FDA, establish the parameters for future submissions," Chief Legal and Regulatory Officer Kathy Hibbs wrote on the blog. "More importantly, for our customers, it marks a baseline on the accuracy and validity of the information we report back to them. The submission includes robust validation data covering major components of our product such as the genotyping chip, software and saliva kit."
According to Engadget, British availabilty won't be a workaround for Americans looking to get the benefits of the system -- 23andMe will only ship to a UK IP address and a UK shipping address. And although the MHRA is not requiring premarket approval like the FDA, the organization is keeping an eye on 23andMe.
"MHRA have asked 23andMe to put in place an enhanced vigilance system in order to gain further reassurance of the performance of the devices in use," the MHRA spokesperson told MobiHealthNews. "This involves the company giving much more information about the safety and performance of the product after it goes on the market in the UK."
Ultimately, though, the British approach seems to put the onus on the consumer to not make medical decisions without consulting with a physician -- an approach a number of US commentators might agree with.
"The information provided by 23andMe should not lead you to make any changes of medical significance without first consulting your healthcare professional," MHRA wrote.















