 Naperville, Illinois-based PhysIQ has received an FDA 510(k) clearance for its personalized physiology analytics system. The offering is categorized as a Class II device for patient monitoring under the product code: automated calculation of a summary index based on several individual measured vital sign inputs.
Naperville, Illinois-based PhysIQ has received an FDA 510(k) clearance for its personalized physiology analytics system. The offering is categorized as a Class II device for patient monitoring under the product code: automated calculation of a summary index based on several individual measured vital sign inputs.
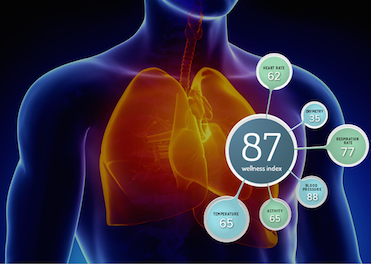
PhysIQ's offering is an early warning detection platform that analyzes a user's physiological data, including heart rate, respiration rate, oximetry, and blood pressure to create a personalized baseline for each user. Rather than comparing a user's physiological measurements to the norms of a population, PhysIQ builds a personalized model for each patient so that the offering can compare a user's data to their own baseline. This method, the company explains, allows PhysIQ to see exacerbations earlier.
“The future of medicine is in the cloud. Real-time visibility into patient condition anywhere and anytime is not a far off dream, but imminent,” PhysIQ Chief Technology Officer Matt Pipke said in a statement. “The key is robust analytics that can distill massive amounts of patient data from wearable and implantable sensors into actionable information for clinicians. Our goal at PhysIQ is to revolutionize personalized digital medicine by empowering physicians to intervene early and effectively to proactively manage disease and improve patient quality of life.”
PhysIQ is building its platform for any organization that is aggregating biometrics from different devices. The platform's workflow is the same, whether it’s monitoring one person or 10,000. The company has already partnered with Samsung on SAMI, which is a platform Samsung created to collect data from various devices. Conkright said at the time that the company is still open to working with other companies that have created similar platforms.
Earlier this year, PhysIQ partnered with the Scripps Translational Science Institute (STSI) to create a program that uses health sensors to improve the health outcomes of Ebola patients. The program collects patient data using Sotera Wireless’ ViSi Mobile System, which continuously monitors blood pressure, pulse rate, electrocardiogram (ECG), blood oxygenation level, respiration rate, and skin temperature from a wearable sensor system. Data collected from the ViSi Mobile System and MultiSense device is sent PhysIQ's personalized physiology analytics platform where the data is analyzed to detect changes in a patient’s health status over time, compared to the patient’s physiological baseline.
Last year, the company raised $4.6 million in a round led by LionBird.



















