 The National Cancer Institute (NCI) has awarded Chrono Therapeutics a Small Business Innovation Research (SBIR) grant of up to $2.3 million for product development of the digital portion of the company's smoking cessation therapy offering. The new grant will fund Chrono's final clinical trial for its product.
The National Cancer Institute (NCI) has awarded Chrono Therapeutics a Small Business Innovation Research (SBIR) grant of up to $2.3 million for product development of the digital portion of the company's smoking cessation therapy offering. The new grant will fund Chrono's final clinical trial for its product.
This is the second grant Chrono has received from the NCI. The first, $2.23 million awarded in 2012, was for early trials and development of the digital therapy. Chrono has also received at least $32.1 million in VC funding from Canaan Partners, 5 am Ventures, Fountain Healthcare Partners, Rock Health, and two strategic investors: GE Ventures and the Mayo Clinic.
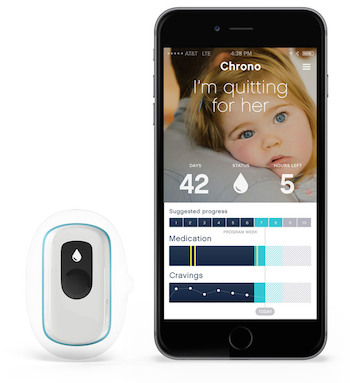
The company’s flagship product SmartStop is a wearable device that delivers nicotine to the wearer at strategic times.
The device is a small adhesive pod worn on the skin. When the smoker starts the program, they choose the initial nicotine cartridge strength based on how much they smoke. Over a period of 10 weeks, the device delivers progressively smaller doses of a nicotine, much like existing nicotine replacement therapy patches. However, rather than releasing the drug continuously throughout the day, the patch will deliver doses at key times, like first thing in the morning and after meals, when smokers’ cravings are most likely to occur. The objective is to not just alleviate cravings, but actually prevent them.
The wearable also connects to a smartphone and delivers information via Bluetooth about whether the user has been wearing the device. That information, in turn, feeds into an app-based behavior change program designed to help smokers cope with cravings. The pod also has a crave button that users can press when they get cravings. This information is sent to the app so that users can draw connections between their cravings, their environment, and their behaviors.
Earlier this year, Chrono Therapeutics said the company hopes to secure an FDA clearance for its device by 2017.
Last year, Chrono CEO Alan Levy told MobiHealthNews that the platform could also be used for delivery of various other drugs for conditions including Parkinson’s, asthma, and ADHD.














