This morning a number of big name medical institutions launched new studies using Apple's ResearchKit, iPhones and, in at least one case, the Apple Watch. As more ResearchKit study apps become available on Apple's app store, it appears that many of them are laying the groundwork for future FDA-cleared medical apps. It not only seems to be the case, the medical researchers behind these apps, in some cases, say that is one of the longterm outcomes they hope for as part of their endeavor.
What's more, Apple itself considers ResearchKit to be a tool that helps those building smartphone-based medical interventions to get them to market. In an interview in New York City earlier this week, an Apple executive explained to MobiHealthNews that this was one of the goals for ResearchKit:
“One way to think about ResearchKit is as the beginning of a pipeline that will lead to more apps that are screening, diagnostic, management and treatment apps,” Bud Tribble, MD, PhD, Vice President of Software Technology at Apple said. "In fact, it is a necessary, essential first step to figure out what is needed to develop these apps -- what works and what doesn’t -- before you move into, ultimately, clinical study apps and clearance to use them for diagnosis and treatment.”
So far 50 researchers have contributed to the development of ResearchKit, according to Apple. Over the course of the past six months these researchers and others have added new features to the open source offerings including: tasks to study tone audiometry for hearing loss; the ability to measure reaction time; PSAT to assess the speed of information processing and working memory, the mathematical puzzle Tower of Hanoi often used for cognition studies; iPad support; image capture; and new participant-facing visuals like pie charts, line graphs and discrete graphs.
Apple also announced that already more than 100,000 people have now signed up to participate in a ResearchKit study.
The three new ResearchKit studies come from researchers at Duke University, Johns Hopkins University, and Oregon Health and Sciences University. Duke has developed an autism study using the platform, while Johns Hopkins has create an Apple Watch-enabled study for people with epilepsy. Oregon Health and Sciences has launched a mole tracking ResearchKit study.
Duke University's Autism and Beyond
 Duke's ResearchKit study, called Autism and Beyond, is looking at the application of a video analysis technology to quantify and analyze the emotions of children so that one day parents may be able to use it as a screening tool for conditions like autism, anxiety, and other behavior related conditions. Autism affects about 1 in 68 children in the US, and while it is possible to diagnose kids at 18 months old, the mean age of diagnosis in the US is five-years-old. In areas with fewer resources the mean age is higher. Duke's team believes that if the study is successful it could someday lead to a screening tool that helps to narrow that gap and get more children help sooner.
Duke's ResearchKit study, called Autism and Beyond, is looking at the application of a video analysis technology to quantify and analyze the emotions of children so that one day parents may be able to use it as a screening tool for conditions like autism, anxiety, and other behavior related conditions. Autism affects about 1 in 68 children in the US, and while it is possible to diagnose kids at 18 months old, the mean age of diagnosis in the US is five-years-old. In areas with fewer resources the mean age is higher. Duke's team believes that if the study is successful it could someday lead to a screening tool that helps to narrow that gap and get more children help sooner.
Autism and Beyond includes a number of short surveys for parents and three videos for children to watch. As the kids watch the videos, which are based on the same kind of stimuli exercises child psychologists use during in-person behavior encoding sessions, Duke's app is using the iPhone's camera to analyze the child's expressions. Parents have the option of sending researchers the recorded video of their child along with the encoded data, or if they'd rather, they can opt to just send the analysis data without the full video recording. The video recordings the researchers do receive will also help them fine tune their algorithms.
"This is not a screening tool," Dr. Ricky Bloomfield, Director of Mobile Technology Strategy and Assistant Professor in Internal Medicine & Pediatrics at Duke University, told MobiHealthNews in an interview. "This is a study to help us evaluate a technology that may one day be used as a screening tool."
The app designers help potential participants understand the time commitments required for each segment of the app by listing the number of questions a survey will have up front or how long each video exercise will last. The videos are all under a minute and the surveys are designed to be quick. In the future the team plans to add more interactive features, like touch-based exercises, that will help them gain additional insights.
Johns Hopkins University's EpiWatch
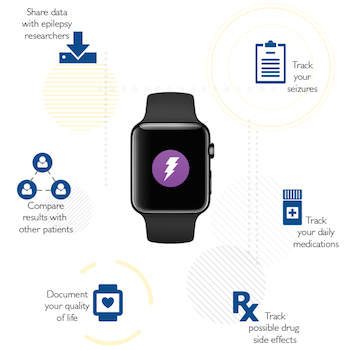 According to the CDC, about 2.4 million people in the US have active epilepsy. About 40 percent have seizures that are not controlled by medications. Seizures are often unpredictable, can occur at inconvenient and dangerous times, and can have a significant impact on a person's lifestyle. For example, those patients who lose consciousness during a seizure can't drive.
According to the CDC, about 2.4 million people in the US have active epilepsy. About 40 percent have seizures that are not controlled by medications. Seizures are often unpredictable, can occur at inconvenient and dangerous times, and can have a significant impact on a person's lifestyle. For example, those patients who lose consciousness during a seizure can't drive.
Researchers at Johns Hopkins University have developed a ResearchKit study that could lead to the development of a seizure detector. Notably, the study, called EpiWatch, makes use of the Apple Watch to collect sensor data they may help to track and sense seizures. For those seizures where movement is an indication, the watch's accelerometer and gyroscope can help track them. For seizures that don't involve movement or convulsions, the person's heart rate still changes. The app will collect this sensor data along with information from surveys filled out by the patient or a caregiver to study duration, frequency, and types of seizures.
"The FDA has a guidance on medical apps that they put out in Feburary," Dr. Gregory Krauss, professor of neurology at the Johns Hopkins University School of Medicine told MobiHealthNews. "The tracking part of this [EpiWatch study] is clearly condoned. The seizure detection research, is research. Participants have consented to provide anonymous research during this study. After a year the question will be: Will we need to file this as a medical device doing seizure detection? This is an evolving area of great interest for us."
EpiWatch participants have access to the data collected as part of the dashboard of the ResearchKit app. The team at Johns Hopkins expects the dashboard to help inform conversations the participants have with their care providers.
Oregon Health and Sciences University's Mole Mapper
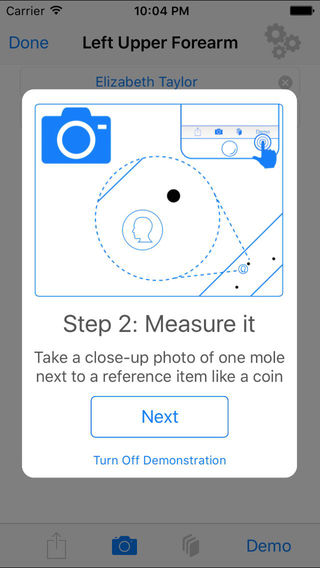 One of the more controversial segments of mobile medical apps has been dermatology, and especially melanoma-focused apps. A handful have had their wrists slapped by the FTC for making unsubstantiated claims over the years, for example. With help from Sage Bionetworks, researchers at Oregon Health and Sciences University have developed a ResearchKit study called Mole Mapper that could help move these apps onto more clinically validated ground.
One of the more controversial segments of mobile medical apps has been dermatology, and especially melanoma-focused apps. A handful have had their wrists slapped by the FTC for making unsubstantiated claims over the years, for example. With help from Sage Bionetworks, researchers at Oregon Health and Sciences University have developed a ResearchKit study called Mole Mapper that could help move these apps onto more clinically validated ground.
In its previous iteration Mole Mapper was a mole tracking app designed by an independent developer, Dan Webster, Ph.D., a National Cancer Institute post-doc, who developed it for his wife and then made it available for free on the app store. After meeting one of the heads of Sage Bionetworks at a conference, Webster was introduced to the team at Oregon Health and Sciences University, which led to the relaunch of Mole Mapper as a ResearchKit study this week.
The app helps users keep track of the moles on their body by having them take photos of them with their phone, assigning them to a region of the body and auto-generating a funny name for them, like Moley Cyrus. For moles the user is concerned about, the app encourages them to take a photo with a dime, which allows the app to determine the size of the mole and if it is changing over time. Since the app knows the exact dimensions of the dime it can figure out the size of the mole despite the photos of the mole being from slightly different angles or distances.
The app's dashboard includes percent change for the moles the user is measuring and it ranks the moles from biggest percent change to least. One of the questions the researchers have is whether that quantitative measure -- percent change -- is enough of an impetus to encourage participants to visit their dermatologist to have a mole checked out.
Study participants also receive monthly surveys that ask whether they have had a mole removed and if so could they submit a photo of the site. Based on how big the wound or scar is, researchers can make an educated guess as to whether the provider removed a mole they believed to be malignant or not.
Five other recent ResearchKit study apps
The five apps that have launched in October include University of Southern California's Biogram; one from microbiome startup uBiome; Hand in Hand from The University of Nebraska Medical Center; C Tracker from Boston Children's Hospital; and the Yale Cardiomyopathy Index.
University of Southern California's Biogram 2
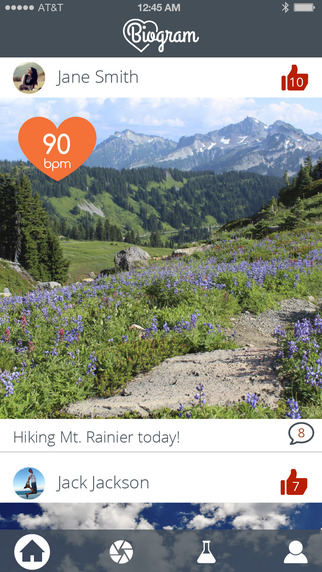 This February, the University of Southern California Center for Body Computing (USC CBC) and app development platform Medable launched Biogram, an Instagram-like app that lets users share their heart rate along with photos. Now the new version of the app will include a ResearchKit study looking into "how publicly sharing biometrics, such as heart rate, influences personal relationships and experiences in a social community."
This February, the University of Southern California Center for Body Computing (USC CBC) and app development platform Medable launched Biogram, an Instagram-like app that lets users share their heart rate along with photos. Now the new version of the app will include a ResearchKit study looking into "how publicly sharing biometrics, such as heart rate, influences personal relationships and experiences in a social community."
The previous version of Biogram used the Alivecor ECG as the preferred means for entering heart rate, but also integrated data from HealthKit. The new version relies primarily on HealthKit to collect the health information -- including heart rate, step counts, and weight -- that the user can include with their photo posts.
"Wearable devices and apps realize the full potential when the user has an emotional connection and even dependence with the technology – it becomes contextualized biometrics leading to greater empowerment and education about an individual’s health and behavior,” Leslie A. Saxon, MD, founder and executive director of the USC Center for Body Computing, said in a statement. “As a researcher, one of the advantages of developing Biogram using ResearchKit is that we can now not only scale our medical studies to capture possibly millions of participants and give us groundbreaking observations but because ResearchKit is an app platform where we can engage users socially and emotionally with their health and study it.”
Medable assisted with the design of the app and will help USC process the large data sets that the study hopes to produce.
Although Biogram is just now adding ResearchKit to its app, the goal of having Biogram be a social media platform that doubles as a way to create large health datasets has been part of USC CBC's mission for some time. Saxon explained Biogram on stage at an event in 2013.
"Now, capturing my heart rate is a way to show you how I’m feeling, is another window to understanding, to enhance, this experience of photo sharing," she said at the time. "And it’s much more of an aesthetic personal understanding, community understanding experience. But at the same time, you’ve suddenly got 10 million 17-year-old heart rates that you’ve archived. These kids have a virtual electronic health record. … It’s a totally different experience than going to the cardiologist and getting your heart rate [measured] or anything else."
Microbiome startup uBiome's ResearchKit app
uBiome CEO Jessica Richman is a longtime proponent of citizen science and uBiome's business model -- in which the company sends test kits out to individuals and delivers them insights about their microbiome -- is based around the same. So it's not a huge surprise that the startup has embraced ResearchKit, which allows them to reach a huge number of people.
"When we heard that Apple came out with ResearchKit, I knew we had to do a ResearchKit app, because pretty much our whole reason for existence is to commercialize ideas for the microbiome using citizen science and ResearchKit is all about that," Richman told MobiHealthNews.
uBiome is using ResearchKit to study the interaction between the microbiome and weight management -- when people gain weight, lose weight, and when their weight stays the same. The app will present users with a survey about their eating and exercise habits. Completing the survey and enrolling in the study gives them the chance to get a uBiome testing kit either free or half-off. Then, the app will connect with smart scales, activity trackers, and diet tracking apps like MyFitnessPal to passively collect data about the subject's weight management.
"We chose that because the microbiome score relates to dozens of different health factors and habits, but we thought weight was something everyone could relate to because we’re all either losing, gaining, or maintaining our weight by definition," Richman said. "So our idea was to do a study that had a broad popular appeal."
Once participants have a test kit, they can take a microbiome sample (essentially a Q-tip swabbed across their toilet paper, Richman says) either once a month or only after a major change in their diet or lifestyle. uBiome will analyze the sample in a lab and send the results back to the patient, as well as using that data in the study.
Traditional research has already shown a link between weight and the microbiome -- in one study a fecal transplant from a lean rat was able to make an obese rat lean, for instance.
"Bacteria seem to be correlated not with your BMI – how underweight or overweight you are – but whether you’re gaining or losing weight; the direction or velocity, not the position," Richman said. "It’s an interesting question whether or not we can make that connection more clear with HealthKit data when we know exactly what people are eating, we know what macro nutrients are there, we know how fast they’re losing weight, because people enter their weight through HealthKit. They actually have smart scales that can feed right in. So think there’s a lot of potential there for new understanding of this data and answering questions that have been sort of plaguing the microbiome for a long time."
Richman said that they had to redesign the app after Apple raised some questions about the user-friendliness of the design. They emphasized the importance of educating users about the microbiome as well as securing data from them, Richman said. Now that this app is out there, uBiome is already thinking about launching more.
"I think citizen science may be an idea whose time has come," Richman said. "It’s actually quite an old idea – it was coined in the 1970s by ornithologists. So it’s not a new idea, but I think the internet and crowdsourcing make it possible on a scale that wasn’t possible when it was birders taking notes and mailing them to ornithologists -- which is awesome, but it’s not like an iPhone app that 11,000 people can download."
The University of Nebraska Medical Center's Hand in Hand
 Hand in Hand, a study developed at The University of Nebraska Medical Center, will recruit patients with HIV to research HIV-associated neurocognitive disorders, aka HAND.
Hand in Hand, a study developed at The University of Nebraska Medical Center, will recruit patients with HIV to research HIV-associated neurocognitive disorders, aka HAND.
Currently, a number of clinics have conducted neuropsychologic testing with people who have HIV, Howard Fox, senior associate dean of UNMC research and development and a professor of pharmacology and experimental neuroscience, told MobiHealthNews. But these tests take approximately 4 hours and must be performed by specialists, who are often PhD-level professionals with a lot of training in administering the tests.
Fox and his partners at Digital Artefacts decided to use ResearchKit to perform cognitive testing that's less time intensive and link that data with other health factors that patients can track into the app, like activity and lifestyle data. Fox said he plans to start with ResearchKit, but one hope he has is that in the future, people can start pairing these results with EHR data to get an even better picture of how HIV can be handled as a chronic condition.
"Our goal is to find what correlates with neurocognitive change and functional decline in those affected with HIV and to be able then to prevent or reverse it," Fox said.
The app will offer a handful of cognitive tests, including memory tests, learning tests, and attention tests.
Fox explained that researchers still have a lot to learn about the disease. One example of this, he said, is that in the past, there was a theory that anti retroviral drugs needed to go to the brain to treat virus in the brain. But researchers later found that at least one of the drugs that goes to the brain itself correlated with neurocognitive dysfunction, so it may not be the virus, but instead the treatment that's affecting it.
He added that researchers also do not know what to expect with the disease, as opposed to other, more researched conditions, like type 2 diabetes, which have certain known consequences, including loss of limbs and blindness.
"This is all new, we didn't think 20 years ago that people would be aging with HIV," Fox said. "And now they are so we can theorize all we want in the lab and in the clinic and we can wait till something happens and then study it. But the more data we can collect through instruments such as this mobile device, and then being able to leak this data to medical records, we may be able to find some new aspects that may be affected. And we may be able to proactively prevent those complications as people age with HIV."
The initial study will run for one year so that the researchers can collect longitudinal data and repeat data. At that point, based on the results, they will decide whether to continue the study, but Fox is optimistic about how the study will play out.
"I anticipate that this will be successful and we'll get some great data and we'll be able to expand this app," He said. "And really, the intention of the app would be research as well as improving people's health."
He aims to capture between 1 and 10 percent of the population that has HIV, which is about 1.2 million, to make the study successful. People have already started enrolling, but Fox said he plans to roll it out slowly. Eventually, once enrollment picks up, he will start working with HIV clinics to get their subjects signed up.
Boston Children’s Hospital's C Tracker
![]() Boston Children's Computational Health Informatics Program (CHIP) released a ResearchKit app last week, called C Tracker, that aims to study chronic hepatitis C, which is a liver disease.
Boston Children's Computational Health Informatics Program (CHIP) released a ResearchKit app last week, called C Tracker, that aims to study chronic hepatitis C, which is a liver disease.
The app allows participants to track their health, medication use, and quality of life. The study is primarily looking at how hepatitis C affects a patient's life.
"In general it's part of a shift from a traditional clinical trial, which tends to focus on specific indicators of disease progression or focuses on perhaps a biomarker measured in the blood, to a study of how patients actually feel, what they function like at work [as well as] at home, and how the disease is having an impact on what they are trying to do with their lives," Ken Mandl, director of the computational health informatics program at Boston Children's Hospital, told MobiHealthNews.
This type of study, which focuses on what's often referred to as patient-centered outcomes, Mandl said, is part of what the active patient community is asking for.
"They are asking that there be a focus on the aspects of their lives that matter to them most," he said "And understanding which medications should be paid for, what therapeutic avenues are most useful, how impactful a disease is on, again, aspects of patients lives that matter most to them. The patient’s voice has largely been missing from most of the design and the focus of clinical studies."
Data from the study is also being integrated into an open source platform that was developed with the help of Boston Children's, which allows researchers to use existing clinical data for discovery research. The framework, called i2b2, which stands for Informatics for Integrating Biology and the Bedside, collects electronic health record data, connected device data, payor claims data, and other data that helps researchers characterize and follow patient cohorts. According to Mandl, i2b2 is in use at over 140 academic medical centers.
In C Tracker, patients who agree to release their data to i2b2 will remain completely anonymous, but in next phase, patients will be able to give the researchers permission to store the data alongside their EHR data.
Though the clinical trial has just begun, one of the first challenges the researchers are expecting is enrollment and sustained engagement with the study.
"The first set of apps was announced on the stage with Tim Cook and so that was some nice publicity for those apps," Mandl said. "Not every ResearchKit app will get that kind of attention. What we’re going to try to do here is work out, in real world, how to conduct these kinds of studies."
Boston Children’s is working Wondros, the company that helped them build their promotional video, to develop strategies to keep patients engaged. The researchers have also partnered with two membership societies, American Association for the Study of Liver Diseases and the Global Liver Institute, to spread the word to physicians and patients.
Yale School of Medicine's Yale Cardiomyopathy Index
Yale launched a study for people who have or may develop cardiomyopathy, a genetic disease of the heart muscle, on ResearchKit earlier this month.
The iPhone-based study, called the Yale Cardiomyopathy Index, was developed by Yale School of Medicine researchers E. Kevin Hall and Dr. Michele Spencer-Manzon. Participants will take self-assessments about their quality of life and heart-related symptoms. They also have the option to perform six-minute walk tests, on an iPhone 5s or higher, that analyze their physical abilities and heart rate trends. The app also offers educational materials about the cardiomyopathies.
The study is open to anyone between the ages of 2 and 80.
"I run the pediatric heart failure and cardiomyopathy group here and my training is in pediatric cardiology so it was always goal for us from the beginning to approach and get enrollment safely, and securely, obviously, from pediatric age groups," Hall told MobiHealthNews.
For anyone under 18, parents will take part in the study with their children and for participants aged 2 to 7, parents will take part in the study on their own, answering questions for their children. These younger age groups are given a different set of surveys that aim to identify how a cardiomyopathy affects them.
"My take, after having been in this field for a while now, is we see a lot of these patients and it’s a relatively common condition in the sense that some types of cardiomyopathies we believe are out there in as many as 1 in 500 people," Hall said. "But that 15 minute window into their lives that we have when they come to a clinic visit is very artificial. I think it’s very apparent that the vast majority of their time and their daily lives is spent far away from the physician’s office. And this is our attempt to better understand the sum total of what their quality of life is or how they view their quality of life at all those points that they’re not sitting in front of us."
In part because cardiomyopathy is a genetic disease and also because he wants to get as many people enrolled as possible, Hall's team added a feature that allows a number of family members to create difference accounts in the app, on one device.
"We felt that was important because once you have one interested person, by nature in a genetic disease, youre also going to have possibly other people in the same family that can contribute," Hall said.
Other ways the study is trying to keep the participants engaged is by providing educational materials so that the app is also a place they can learn about the condition. But even if people don't stay engaged with the app for a long period of time, Hall said the study will still benefit from some of the data at least.
"There’s two dimensions of data," Hall said. "If we had 1,000 people sign up, fill out all the surveys, and never come back again, at least we have point in time data for these users. Our goal, though, is to do this over the course of the year a number of times, and so we hope to not only have point in time data but temporal data as well to see how these things are changing over time."
One challenge for researchers, however, is that they are legally limited in giving back direct medical advice, which is different from a study that someone might enroll in with their own doctor, which would allow them to contact their doctor if their condition changes.
This summer, just one app launched: PRIDE from University of California San Francisco.
The University of California San Francisco's PRIDE
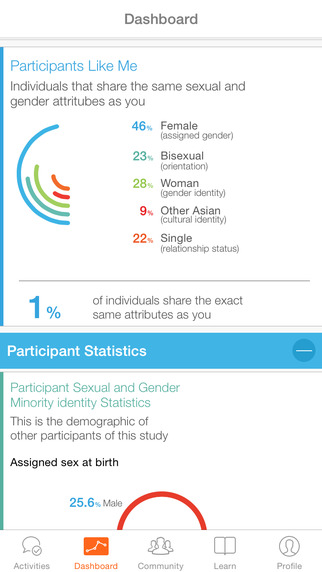 The University of California San Francisco launched a longitudinal study of lesbian, gay, bisexual, transgender, queer (LGBTQ) and other sexual and gender minority (SGM) adults in June to examine how their sexual orientation affects their health. The study, called PRIDE, stands for population research in identity and disparities for equality.
The University of California San Francisco launched a longitudinal study of lesbian, gay, bisexual, transgender, queer (LGBTQ) and other sexual and gender minority (SGM) adults in June to examine how their sexual orientation affects their health. The study, called PRIDE, stands for population research in identity and disparities for equality.
Dr. Juno Obedin-Maliver, a co-director of the study, told MobiHealthNews that they first found out about ResearchKit with the general public when it was announced and started working on the ap almost immediately. Prior to the announcement, they had been working on the PRIDE study for around a year and half with the intention to make it a web-based platform eventually.
"We are really excited about what is happening with the research," Obedin-Maliver said. "Basically we didn’t expect as big of a response as we’ve had. We’re at almost 15,000 participants at this point, just shy of that. The prior studies on LGBT health were in the 5,000 to 6,000 range. In just about four months, we exceeded that almost by triple. It's much bigger than prior studies."
When the study first launched, researchers explained that they would start by asking participants to express their health concerns so that researchers can then crowdsource health topics that are important within the LGBTQ community.
"Unlike the other ResearchKit apps, we have a community forum, where people can propose and prioritize research questions in conversational settings, much like Reddit, where you can propose comments, people can comment on those threads, and they can vote them up or down," she said.
In just the three and a half months since PRIDE launched, participants have contributed 4,000 unique threads, but since the researchers can't address all of those questions, they are looking to connect the threads that fall under similar themes and incorporate that into how they are thinking about the questions they ask. The study coordinators will also lean heavily on their partners in LBGT community, representatives of service organizations, healthcare clinics, and advocacy organizations to get an understanding about what these organizations are seeing on the ground.
"Very early on we heard a lot about the interest in comprehensive health including mental, physical, and social health," Obedin-Maliver said. "Particularly, there was a lot of interest around mental health both in terms of things like depression and anxiety but also in terms of resiliencies -- the unique, strong features of the LGBTQ community. In response to that, and partly because we had a sense this was going on, we have a whole mental health team. In our next surveys that we are planning to push out, we have dedicated time to each of those topics: physical health, mental health, and social health."
So far, one of the biggest challenge they have faced in working on the ResearchKit framework is that participants cannot receive new surveys without going through an app update, but Obedin-Maliver added that Apple and the ResearchKit team are working on this issue and are actively working to fix it.
"It's only a challenge in so far as the promise and the potential of ResearchKit and other technologies like this is that we have a more immediate way of being in touch with our community," she explained. "Before it used to be that people would send out letters and phone calls and put up posters in community centers and then wait for people to call. Now we have a much more immediate way of being in touch with people and it’s almost like we want even more immediacy."
In terms of results, Obedin-Maliver aims to get a diverse group of people across the country involved in the study, including people with different ethnicities and from different areas around the country. She also wants the study to be worthwhile for the participants.
"Are they staying engaged?," she explained. "Is the engagement meaningful to them? Do they feel represented? Do they feel like their voices are being heard? And are we answering questions that nobody else has tried to or has been able to answer?"
Updates on three of the first five ResearchKit apps
MobiHealthNews was able to catch up with three of the original five ResearchKit study apps. Researchers working on GlucoSuccess and MyHeart Counts were not able to make themselves available for comment. GlucoSuccess is a ResearchKit app developed at Massachusetts General Hospital that is crowd-sourcing a database of health behaviors and glucose values for people with type 2 diabetes. MyHeart Counts, a study app developed at Stanford Medicine, aims to improve their understanding of heart health.
The Icahn School of Medicine at Mount Sinai's Asthma Health
Asthma Health is the only app -- of the first five that launched with ResearchKit -- that has made any big updates to its study since launching. Last month, The Icahn School of Medicine at Mount Sinai and LifeMap Solutions, two of the organizations that developed the app, announced that they added a new feature, called Doctor Dashboard, which will push the app beyond the realm of research and into clinical use.
Asthma Health aims to help patients adhere to their treatment plans and avoid asthma triggers. Patients can use the app to record daytime and nighttime asthma symptoms as well as how they affect the patient’s activities. It also tracks daily usage of controller and rescue inhalers along with asthma triggers: colds, increased physical activity, strong smells, exhaust fumes, house dust, peak flow, and animals. Finally, it tracks emergency department visits, medical visits, and changes in medication. The app will also send updates about when users should take medication and what the air quality is like in a specific location.
Since launching Asthma Health, six months ago, the researchers recruited and enrolled over 8,600 research participants. While the app was launched as a research tool, Yu-Feng Yvonne Chan, director of digital health and personalized medicine at the Icahn Institute at Mount Sinai, who is also a lead researcher on this study, explained that after using the app, study participants told researchers that the app was also helping them better understand and manage their condition.
"With the recent announcement of new features, we definitely see a re-spike of some of some of these numbers so that's something we are trending right now," Chan said. "It’s too early to show the actual impact, it could be short lived, but that does speak to the nature of the beast with these types of studies."
Chan explained that she expected a significant number of participants to drop off so the percentage of people who have continued to use the app surpassed their expectations somewhat. Generally, when a study like this one first launches, there is an initial influx of a lot of curious folks who just want to try it out and see what the hype is about, who are not be motivated to stay on.
For Asthma Health, Chan said that of the 8,600 people who have enrolled, just a few thousand are most useful in terms of providing enough ongoing data for researchers to really study and analyze what patterns exist. The data is already showing somewhat of a trend from the first six months.
"When a patient first enrolls in our study, we ask a bunch of questions about the baseline -- how well their asthma is controlled -- and some questions which speak to the severity of the disease, their asthma," Chan said. "And it seems to us that the patients who have more poorly controlled asthma from the get go and those who seem to have more severe disease, seem to stay with our study more consistently, for a longer period of time. One of the assumptions we are starting to make is that perhaps, it seems to us that that particular subset of patients must perceive value in using this app and therefore they are more motivated to stay with the study and continue on participating. That’s one area we are very interested in flushing out more."
The Asthma Health researchers are also planning to continue building features that will engage the patient further -- Doctor Dashboard was just the first.
"The first half a year since the start of recruitment, we have been really fixated on making sure the research was done correctly," Chan explained. "Now that we are at the six months mark, and all those things I mentioned are squared away, as a team, we’ve had multiple discussions over the last few months that tackle the engagement issue. As an institution as well as the scientific community and medical community, we are definitely heading more towards the consumer, patient-centric type of practice. It’s about the consumer, and that’s where a lot of our answers lie."
Since the team refocused on engagement, Asthma Health has put together a consumer panel that they can consult on adding new features. The team, Chan said, already has an "elaborate excel spreadsheet of the next features" that they plan to roll out although these decisions will also be based on how resource heavy the features are for Asthma Health's technology partners.
"This is just one example of how we will be able to modify our app to meet the needs and wants of participants," Chan said. "At the end of the day the app has to serve a very tangible need. You’ve got to help the person do something better, more efficiently, more enjoyably."
Dana-Farber Cancer Institute's Share the Journey
Not every ResearchKit app has been a resounding success. Share the Journey, an app which aims to analyze why some breast cancer survivors recover faster than others, why patients’ symptoms vary over time, and what can be done to improve their symptoms, has apparently faced some problems with retention.
"Our sense was that we probably made a very complex app, very demanding with a lot of daily tasks and focused on a patient population that already finished treatment that probably just wanted to go back to the life without thinking about cancer anymore," Dr. Ines Van Luis, a researcher in the Breast Oncology unit at the Dana-Farber Cancer institute and a consultant on the study, told MobiHealthNews.
Van Luis referred MobiHealthNews to Sage Bionetworks for the exact numbers on enrollment and retention, and Sage didn't respond to requests for that information. But Van Luis strongly implied those numbers weren't at an ideal place. But that represents a learning in and of itself -- that ResearchKit is a powerful tool, but it has to be aimed at the right populations and tailored to those populations.
"We have to reduce some of the complexity to make the app more friendly," Van Luis said. "I think there were like a reasonable number of patients that downloaded the app. There were some patients that seven months out continue to give data, but we also have a sense that we have to communicate with the patients to be able to engage them more."
Sage is analyzing the data that's been collected so far, and researchers are still hopeful that will return some insights into the original questions of the research. But they are also working on refining their approach for a follow-up app, that might focus on patients undergoing active treatment, rather than on survivors.
"We are just in the beginning of the journey and we will have to conciliate this new tool with the way we used to do research and learn to engage patients, retain patients, and maximize the way we can utilize this kind of tool," Van Luis said. "I think that’s basically where we are now."
The Beijing Institute of Geriatrics, The Michael J. Fox Foundation for Parkinson’s Research, Sage Bionetworks, and the University of Rochester's mPower
 Different diseases lend themselves to ResearchKit for different reasons, but the platform is peculiarly suited to studying Parkinson's Disease, and according to Dr. Ray Dorsey, the neurology expert for the mPower study, the early results have been very promising.
Different diseases lend themselves to ResearchKit for different reasons, but the platform is peculiarly suited to studying Parkinson's Disease, and according to Dr. Ray Dorsey, the neurology expert for the mPower study, the early results have been very promising.
"We’re seeing how people do on Saturdays and Sundays, how people do between 5 pm and 8 am," he said. "Heretofore we’ve had no good way of measuring that because the only way has been to get people into clinics and have them stay for hours. Now we can assess people’s symptoms across the course of the day and identify things that make their disease better or worse, or see the response to exercise or treatment. So I think we’re getting a lot of data that’s high frequency, that’s likely to be more sensitive than traditional measures and more objective than traditional measures. In many ways this is filling in large gaps in the way we currently assess Parkinson’s."
The mPower study is being jointly conducted by the Beijing Institute of Geriatrics, The Michael J. Fox Foundation for Parkinson’s Research, Sage Bionetworks, and the University of Rochester, with which Dorsey is affiliated. People with and without Parkinson's Disease can download the app and it will passively track their gait and movement using the phone's accelerometer. Participants also take part in tap tests throughout the day that test their fine motor skills. The study has seen 14,000 enrolled, and early analysis of the data demonstrates how much information is contained in those tests.
"We can differentiate individuals with Parkinson’s versus those without, we can detect pharmaceutical response to treatments, so we can [distinguish] that individuals before and after they take Levodopa for their Parkinson’s. We’ve picked up other measures of tapping, like rhythmicity and accuracy also improve, and we’re getting feedback. We’ve gotten around 20,000 suggestions from participants on how to improve the app and what’s making people better or worse including things we’d never previously considered, such as weather."
Dorsey couldn't give exact figures on retention, but he said it's been good, all things considered.
"Like with any app, there’s rapid adoption and decay in use. And we’ve seen that. Decay is greater amongst those without that disease, but among those with the disease there’s been a decay but there’s more retention," he said. "Remember this is the first version. We think that future versions could have even greater value and greater power to participants especially when we address these 20,000 concerns. We can look back and see what are the drivers of engagement, drivers of use compared to what are the factors that lead to lack of use."
The group's first publication, an overview of some of the lessons learned, is due out in a few months. But the researchers aren't planning to keep the data to themselves; 70 percent of participants has opted into having their aggregate, deidentified data made available to a broader group of researchers.
Unlike some of the other ResearchKit apps, mPower includes a novel assessment tool, so their goals extend somewhat beyond the study. Roche has already begun using a version of the app in a drug trial. We previously reported that it was a similar app, but Dorsey confirmed that it's the same software.
In the future, Dorsey would like to see the mPower assessment tool improve patient self-efficacy; help clinicians manage their Parkinson's patients; help researchers investigate drugs, devices, and therapies for treating Parkinson's; facilitate diagnosis of the disease; and even serve as a platform for remote access to care.















