
Last week, as part of our Focus on Innovation, HIMSS Media ran a poll about the FDA's Pre-Cert program. The program isn't new, but we thought it would be a good moment to take the public pulse on a program that has seen a variety of reactions from inside the industry. Just under 500 readers responded.
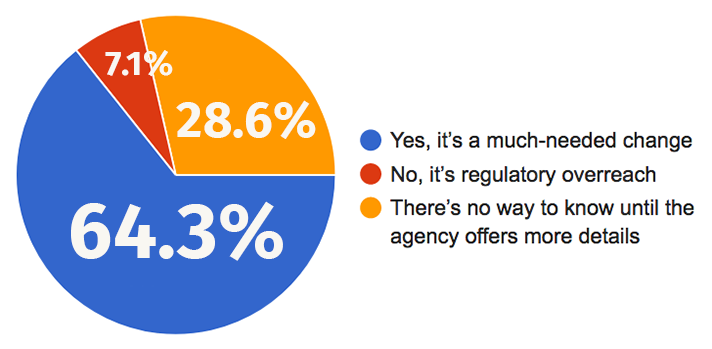
In general, those opposed the program constituted a vocal minority — just 7.1 percent of respondents, many of whom sent in comments. A majority, 64.3 percent, favored the change. And a significant number, 28.6 percent, felt there just wasn't enough information out there to know if the program would be a help or a hindrance to the industry.
Is the FDA’s Pre-Cert program good for the industry?
What we couldn't have anticipated on Monday was that Apple, a member of the Pre-Cert pilot program, would announce its first-ever FDA clearances on Wednesday. Although Wednesday's De Novo clearances were not directly related to the Pre-Cert program, the unprecedented speed of Apple's clearances drew attention to one possible foible of the program — that it could create a hierarchy of "have" and "have not" companies, some of which have a fast track to clearance and some of which don't.
"It is not so much a regulatory overreach, as a way to continue to load the playing field in favor of the big players," one reader wrote. "This will stifle innovation, and the small startups and entrepeneurs are being put in a disadvantaged position before they start. The idea of streamlining the approval process is a good one, but this approach does not work."
Multiple readers took issue with the phrase "regulatory overreach," feeling the program was bad for other reasons.
"It's not regulatory overreach, these devices should be regulated," one wrote. "Its a bad, unfair, poorly defined regulatory program."
The notion that the program was too ill-defined for as long as it has been in development was a popular one among the crowd asking for more details.
"So far the program seems to be based on the principle that 'good' companies can be relied on to deliver good products," one wrote. "Unfortunately, FDA does not have experience or expertise in qualifying companies with cultures of excellence so the approach is likely to be burdensome and uncertain. In addition, it is easier to identify 'bad' products post-market but the agency has not articulated how it will create an efficient mechanism to do so."
Others wanted to know what FDA would do to make the system fair, even in the presence of bad actors.
"It only works with ethical companies that can and are willing to utilize good systems," one reader said. "Small companies won't be able to meet the expectations and unethical companies will ruin it for the rest."
But again, the fans of the program dominated the poll. For these readers, Pre-Cert represents hope for a long-awaited regulatory scheme for digital health that can move at the same pace as digital health innovators.
"The FDA should be an enabler of innovation. Love this," one wrote.
"A high-quality health monitoring product can come to market earlier as pre-certification programme will probably be faster with partial assurance about product quality," said another.
For many, the idea of an "Excellence Certification" rang true in the face of an industry they felt was suffused with sub-par products.
"I think (hope) it will keep out the phonies and the wannabes and act as a certification of valid and reliable products," one wrote.
"Certification should mean that certain standards are being met for specified safety and purpose," wrote another. "There are products on the market that do not do what is marketed as their function and this is deceptive and can cause harm to the ignorant buyer."
Focus on Innovation
In September, we take a deep dive into the cutting-edge development and disruption of healthcare innovation.















