 After more than five years of designing, building, and testing versatile, portable consumer medical devices, the $10 million Qualcomm Tricorder X Prize has announced a winner. Final Frontier Medical Devices, a small team led by engineer-turned ER doctor Basil Harris and his brother George (also an engineer) won the top prize of $2.6 million. Runner-up Dynamical Biomarkers Group will walk away with $1 million.
After more than five years of designing, building, and testing versatile, portable consumer medical devices, the $10 million Qualcomm Tricorder X Prize has announced a winner. Final Frontier Medical Devices, a small team led by engineer-turned ER doctor Basil Harris and his brother George (also an engineer) won the top prize of $2.6 million. Runner-up Dynamical Biomarkers Group will walk away with $1 million.
Walk away may not be the right word, however — the X Prize foundation also announced the ways it will continue to support these teams and the other five finalists in bringing their tricorder devices to market, even though the competition is officially over. The balance of the $10 million (minus $1 million given out as milestone prizes over the course of the competition) will go to funding these initiatives.
“We’ve won already,” Basil Harris told MobiHealthNews in an interview earlier this week, before the winner was announced. “This is a win across the board. No matter what the final announcement is, we feel like for my team this has been incredible.”
“Every team that was able to get into the top seven that came out with a device are winners,” DBG team leader Chung-Kang Peng added.
A long road
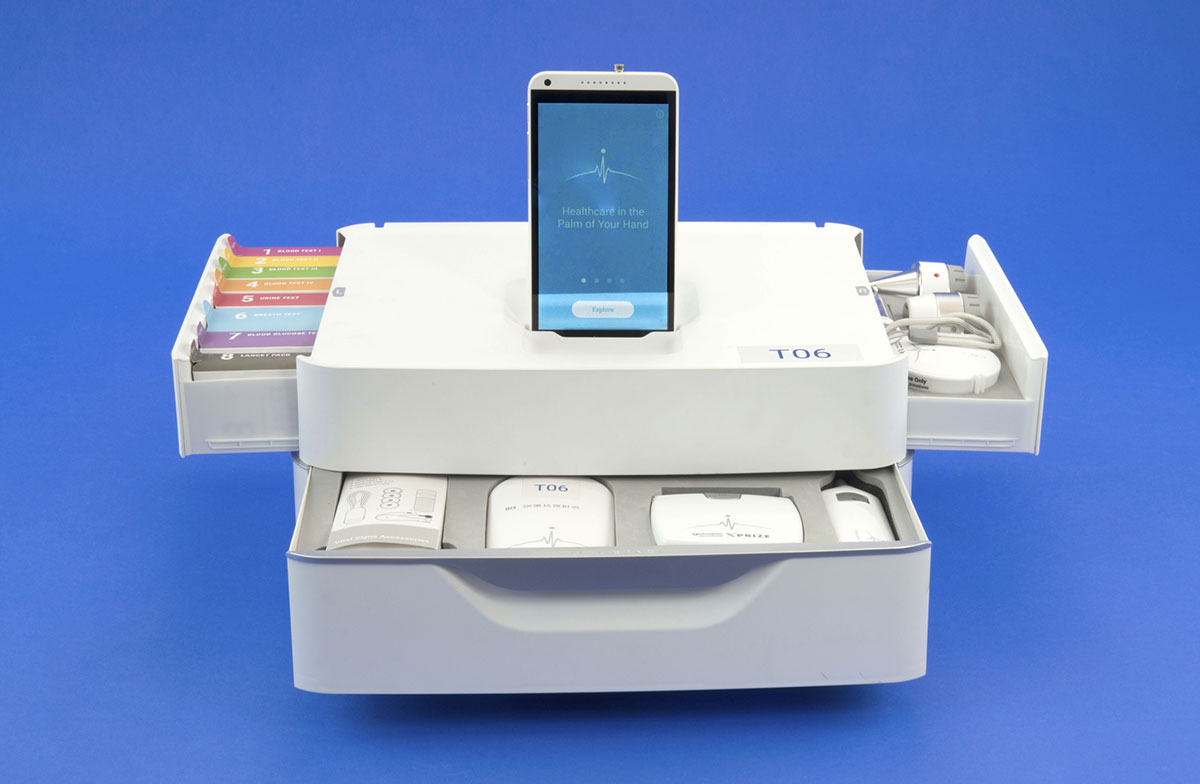
Qualcomm and X Prize launched the prize at CES in 2012, licensing the word tricorder from CBS — the term originated as a handheld scanning device, most memorably a medical scanner, on Star Trek. Teams were challenged to build a real-life version of the tricorder, a device weighing five pounds or less that could continuously monitor five vital signs, diagnose 13 disease states, and be used not just by a medical professional but by anyone.
“It’s like when they thought up the competition they said ‘It’s not hard enough to make a tricorder. Let’s make a tricorder and take Bones McCoy out of the equation too,’” Harris joked.
Tricorder X Prize Director Grant Campany said that all X Prizes, starting with the Ansari X Prize for Suborbital Spaceflight that launched in 1996, aim to nudge the market forward in areas where it might not move or might not move fast enough on its own.
 “We focus on what we consider to be bottlenecks or market failures,” he said. “One of the most profound market failures facing mankind today is that hundreds of millions of people are without modern healthcare. And the opportunity [to fix that failure] is technology. … If X Prize didn’t step in, where would money come from to support R&D and testing for devices that have never been developed before? Given the relevant risk, this is where X Prize sees its sweet spot. How can an incentivized competition really tip the curve and really provide the impetus for development from people around the world — not just geographically limited but make it a truly global operation where we can attract the best minds and the best talents to this particular problem.”
“We focus on what we consider to be bottlenecks or market failures,” he said. “One of the most profound market failures facing mankind today is that hundreds of millions of people are without modern healthcare. And the opportunity [to fix that failure] is technology. … If X Prize didn’t step in, where would money come from to support R&D and testing for devices that have never been developed before? Given the relevant risk, this is where X Prize sees its sweet spot. How can an incentivized competition really tip the curve and really provide the impetus for development from people around the world — not just geographically limited but make it a truly global operation where we can attract the best minds and the best talents to this particular problem.”
The original call for submissions attracted more than 300 pre-registrations. By late 2013, when the X Prize announced the teams for the first time, there were 34. The diversity of the teams was impressive — from Silicon Valley startups to large public companies to garage operations, there was really no common thread. They came from all over the world. There were college teams (one of which made it to the semifinals) and even a high school team.
By the end of 2014, the 34 teams were winnowed down to 10 semifinalists, which eventually became seven after two teams merged and two dropped out (one by choice and one because its prototype was tragically held up in customs). These 10 built prototypes and had to demonstrate their accuracy in clinical tests.
At the end of last year, only DBG and Final Frontier remained to move on to the final phase, where the devices were left alone in a room with consumer testers, to see if they were sufficiently user friendly.
The final two
Final Frontier Medical Devices and Dynamical Biomarkers Group couldn’t be more different. The Washington Post called the end of the competition “A Basil and Goliath Story”: Harris’s team consisted (at least originally) of seven people working nights and weekends with next to no budget, whereas Peng had 50 doctors, scientists and programmers and the backing of HTC and the Taiwanese government.
At the end of the day, though, the teams produced devices that are similar in many ways. Both DBG and Final Frontier ended up with modular devices that include a touchscreen (a smartphone for DBG and a tablet for Final Frontier’s device, called DxtER) and a number of different sensors. And both teams had the same learning curve about the importance of the user experience aspect.
“We came at the problem a little bit differently I think than most of the other teams that entered the competition in that I came at it from the AI perspective of ‘What do I do in the ER to make a diagnosis?’,” Harris said. “…I think we did it in the reverse direction of most teams that may have started with the cool sensors and then built the AI afterward. I think you need both components, but we’re a small team, so we started with what we knew. I think that sums us up from a design perspective.”
Peng agreed, saying that sometimes it was difficult to get the UI and clinical parts of the team on the same page.
 “We initially naively thought it was purely a technology problem,” he said. “We developed algorithms for image analysis, for signal analysis. Like Basil said it’s a completely different approach. At the end, we reached pretty much the same conclusion. The clinical AI needed to help you do the screening, diagnosis, prescreening — those are actually more important.”
“We initially naively thought it was purely a technology problem,” he said. “We developed algorithms for image analysis, for signal analysis. Like Basil said it’s a completely different approach. At the end, we reached pretty much the same conclusion. The clinical AI needed to help you do the screening, diagnosis, prescreening — those are actually more important.”
Harris says his small, close knit team was actually an advantage, as it allowed them to quickly pivot and discard ideas that weren’t working.
“When you’re developing into the unknown like this, we went down many blind passageways and dead ends and you just learn from those mistakes and you regroup,” he said. “You need to decide when to cut off a certain path and go down a new one if it’s not giving you the results that you need.”
Campany also echoed the importance of the direct-to-consumer piece, which gives the tricorder a distinctly different role to play in healthcare than simply an advanced medical sensor.
“I think what really separates these devices from anything else out there is they have been objectively evaluated under strict conditions with the intended user being the consumer,” he said. “I know there’s a lot of companies that claim they are the tricorder, but I challenge any of them to stand up to the rigor or the complexity these devices have been challenged to design. We talk about 13 medical conditions, continuously monitoring five vital signs and intended to be used by a consumer. These are very different criteria than anything else we’ve seen and quite frankly we think these teams are at least 18 months ahead of anything else being developed around the world.”
What’s next?
Now that the contest is complete, the market’s push has been delivered. The question is, will it be enough to keep it rolling?
“I think what we’ve seen now is we have a real demonstration of technology, deployed in an objective environment, and there’s validation there,” Campany said. “When we consider what opportunities that opens up for us, now that there’s data, people will be incentivized to not only collaborate with these teams but also to fund them. We’ve gone beyond the valley of death now and we’re looking at how we can now accelerate the development of these technologies to reach the masses. That’s really what this is all about.”
But that doesn’t mean Qualcomm and the X Prize Foundation won’t give the teams some help.
“This Qualcomm Tricorder X Prize generated a critical mass of diverse and qualified teams, enabling nearly five years of experimentation and iterations, that ultimately resulted in an extraordinary outcome,” Marcus Shingles, CEO of the X Prize Foundation, said in a statement. “We could not be more pleased with the quality of innovation and performance of the teams who competed, particularly with teams Final Frontier and Dynamical Biomarkers Group. Although this X Prize competition phase has ended, X Prize, Qualcomm Foundation, and a network of strategic partners are committed and excited to now be entering a new phase which will support these teams in their attempt to scale impact and the continued evolution of the tricorder device through a series of new post-competition initiatives.”
In addition to the winner, the X Prize announced tonight that the Qualcomm foundation will put $3.8 million towards the “promotion of the digital health ecosystem, including the continuation of consumer testing, guidance for the Qualcomm Tricorder XPRIZE teams, and support for further development of tricorder devices,” the foundation explained in a statement. “Specifically, the Foundation has requested a proposal for a $2.5 million grant to the University of California, San Diego, including the Altman Clinical and Translational Research Institute. The balance of the commitment will be distributed to other organizations.”
The Roddenberry Foundation, established by the son of Star Trek creator Gene Roddenberry, pledged an additional $1.6 million toward the goal of getting tricorder devices into hospitals and communities.
Over the course of the competition, the FDA has worked with the X Prize Foundation to provide a dedicated help desk for participants. The FDA will continue to support the finalists and semifinalists as they move their devices toward the market.
“We formed a relationship with the FDA, the CDRH, a little over three years ago,” Campany said. “The FDA formed this FDA help desk which enabled 18 volunteers to interact with the teams. And now that relationship becomes even more critical as these devices are basically honed for commercialization and receiving the appropriate guidance from the FDA on what they need to do from a clinical standpoint to actually get to market. We think we have a tremendous asset there with the FDA and I think the teams and everyone else who will be using this resource will receive a lot of value from it.”
The X Prize will also produce a feature-length documentary and a traveling exhibit to raise public awareness of the technology, and will work with retailers to secure distribution channels for the finished devices.
What’s next for the teams is more work, but with a loosening of some of the constraints of competition. The teams are now free to work together and learn from each other, something both finalists have considered.
“We’re not stopping,” Harris, whose team has incorporated under the name Basil Leaf Technologies, said. “We’re pushing ahead. It’s not something where we can take this demo product and start marketing it. This is health-related serious business with making diagnoses. We’ve started the process of getting each of the individual components approved through the FDA and that involves clinical testing. The competition was very rigorous and modeled after a clinical trial, but it was still a competition and the FDA wants more data. At my hospital, we’ve started a clinical test with our first novel sensor and we’re going to march through each of the sensors until we can build up the whole kit.”
DBG is working on a second generation device that, freed of the X Prize’s weight limit, will be able to diagnose 50 diseases, not just 13.
“We actually have a plan to deploy this system in rural villages in China,” Peng said. “We’re in discussion with the Chinese government right now.”
Much like Gene Roddenberry’s bright vision of the future on Star Trek, Harris has his own dream of how his device, or ones like it, will be used.
“My dream in the future is to be in the ER and have a patient come in and say ‘I have this tricorder’ and maybe CK’s team made it or one of the other teams made it, it doesn’t matter,” he said. “This is a technology that’s coming down the pipe. I want to be able to believe that information and go to the next step. … That’s where we’re going to start to see an improved efficiency in the healthcare system and improved access to quality care across the board.”
You can read more about the full timeline of the competition here.
















