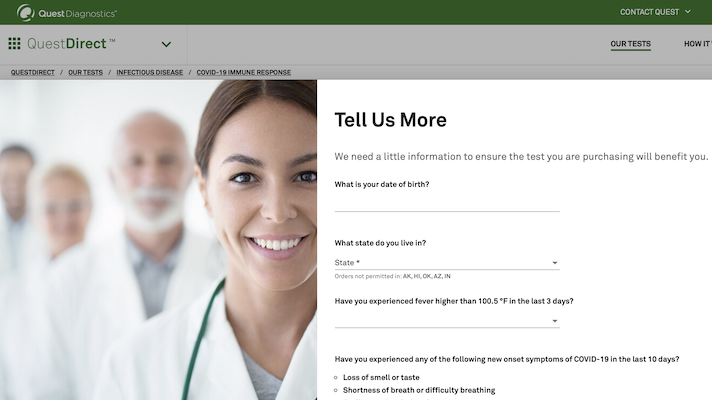
This morning behemoth lab test company Quest Diagnostics announced that it will be offering a “consumer initiated” COVID-19 antibody test for $119.
Dubbed Quest Direct, the service will let consumers request the antibody test through an online platform. Once the request is initiated it is sent to a licensed physician, who greenlights the testing order. Patients can then go to a Quest service center to get their blood drawn, and get results within one to two days via an online portal.
Patients can also tap into telehealth services to discuss their results with a doctor.
The antibody testing will be based on tests from Abbott and EUROIMMUN. At the beginning of this week Abbott landed an Emergency Use Authorization for its serology test. EUROIMMUN is still in the process of submitting for an EUA, although the test is still able to be used in CLIA labs under a March FDA policy. According to Quest, Abbott reports a 99.4% specificity for its antibody test, and EUROIMMUN AG is reporting a 98.5% to 99% specificity.
The tests will be specifically aimed at testing for COVID-19 antibodies, which means the test is not meant to be used to determine whether or not a person is positive for the virus.
When the virus first hits, a patient’s body is still developing antibodies in the early days of the infection. That means that serology tests can provide negative results if administered too early. The other issue is that test results could be positive if a person was once infected with the virus but is no longer ill, according to the agency.
Quest specifies this test is not intended for those who feel sick or who have tested positive for the coronavirus within seven days.
WHY IT MATTERS
In many conditions when a patient develops antibodies, it can mean they have protective immunity. However, the CDC said that at this time it is still unknown if having detectable antibodies for the virus can protect a patient from getting it again or for how long.
However, those with the antibodies are now being recruited to help with clinical trials. Plasma has recently come onto the scene as a potential therapy. The CDC is encouraging people who have recovered from COVID-19 to donate plasma, citing the new national efforts to study convalescent plasma.
Just this morning the UK’s NHS Blood and Transplant organization announced its plans to solicit blood from recovered patients for plasma-focused clinical trials.
THE LARGER TREND
At the beginning of April, Cellex became the first company to land an FDA EUA for a serology test.
However, the tests haven’t been without controversy. The science community has been skeptical of the accuracy of such tests. And in early April, at least one company mistook its response from the agency as an EUA and falsely marketed its service. It wasn't long after that Cellex’s test was released.
But this isn’t the first direct-to-consumer marketing of tests. On April 21, LabCorp’s COVID-19 RT-PCR test became the first diagnostic test for COVID-19 that permits at-home sample collection.


















