 According to an FDA filing, wireless-enabled blood glucose meter developer Telcare just received 510(k) clearance for its device, Telcare BGM. The company submitted its device for FDA review at the beginning of March.
According to an FDA filing, wireless-enabled blood glucose meter developer Telcare just received 510(k) clearance for its device, Telcare BGM. The company submitted its device for FDA review at the beginning of March.
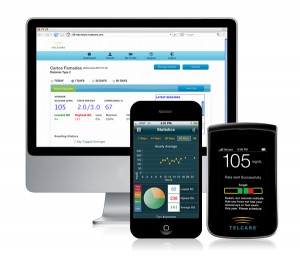
Telcare has largely eschewed the glucometer-smartphone pairing model currently pursued by a number of other device makers in favor of a wireless meter "that requires no additional investment."
Telcare's device is cellular-connected and according to the company it is "merely a replacement of older technology for which insurers already pay."
The Telcare BGM includes 3G cellular connectivity that send glucose tracking data to the company's clinical server. Telcare is also developing a suite of smartphone apps, which might be used by caregivers, including "parents of children with diabetes and those caring for elderly people with diabetes," to better manage their loved ones.
"Moreover, the Telcare blood glucose meter significantly increases the work productivity of disease management personnel who no longer need to spend time calling patients to ask for glucose data and can now relay simple coaching directly on the Telcare blood glucose meter platform instead of using less efficient means of outbound communication,” Telcare states on its website. The company says the device can send data directly to electronic health records (EHRs).
Telcare has previously stated that it plans to offer its blood glucose meter offering to customers at the same pricepoint of “disconnected” devices that are already on the market.
The company has raised about $4.5 million in funding to date from investors including Qualcomm Ventures. It also works with Sierra Wireless for its device's connectivity. (Update: Sierra is not an investor in the company.)
Learn more about the Telcare executive team (led by Jonathan Javitt MD MPH) at Telcare's site.














