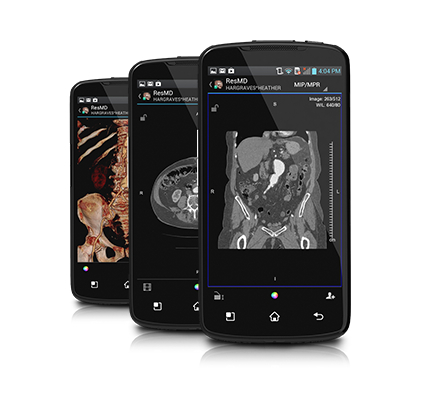
 Calgary Scientific announced this week that the US Food and Drug Administration had cleared its ResolutionMD software for diagnostic medical imaging on Android devices, including the Samsung Galaxy. The software is already cleared by the FDA for iOS devices and by regulatory agencies in Europe and Canada.
Calgary Scientific announced this week that the US Food and Drug Administration had cleared its ResolutionMD software for diagnostic medical imaging on Android devices, including the Samsung Galaxy. The software is already cleared by the FDA for iOS devices and by regulatory agencies in Europe and Canada.
In early 2011 the FDA cleared a competitive offering, called Mobile MIM, as the first diagnostic imaging app to secure FDA Class II clearance for use on iPhones. Calgary Scientific claims that its ResolutionMD software, which now has six FDA clearances -- including for iPhone and iPad back in September 2011 -- this is the first diagnostic imaging app cleared for use on mobile devices, according to the company.
Calgary Scientific has inked a number of deals since it received its first FDA clearance for the mobile version of its diagnostic imaging offering. ResolutionMD is used by neurologists at the Arizona location of the Mayo Clinic for remote consultations, and the company also has deals with Sprint and AT&T.
In 2012 a study at the Mayo Clinic in Phoenix found that radiologists using ResolutionMD Mobile versus a traditional PACS workstation were able to access CT scans of stroke victims 24 percent faster, according to the company. Calgary Scientific says the study found its software saved an average of 11 minutes per patient. This data appears to be new as our report at the time focused on the accuracy of the diagnoses instead of the specific time to diagnosis.
About a year ago a study from the University of Maryland found that radiologists using iPad 2s to evaluate patients for tuberculosis (TB) took twice as long to make a diagnosis as they did when using a 27-inch LCD monitor. However, that study used a different imaging software from Osirix.



















