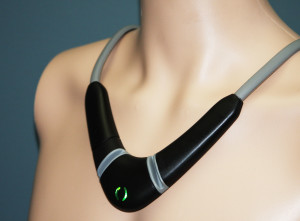
 A startup called Perminova is developing a necklace for clinical use that can track a number of vital signs including thoracic fluid levels, an early predictor of congestive heart failure that isn't currently monitored by most connected sensors. Patients at risk for CHF could use the device in place of or along with a weight scale.
A startup called Perminova is developing a necklace for clinical use that can track a number of vital signs including thoracic fluid levels, an early predictor of congestive heart failure that isn't currently monitored by most connected sensors. Patients at risk for CHF could use the device in place of or along with a weight scale.
"You have weight gain four days before a heart failure event," VP of Marketing Susan Pede told MobiHealthNews from the floor at HIMSS. "About seven days before that you can detect fluid build-up that's trending upward. About 18 days before that you can see changes in stroke volume and cardiac output."
The system, called the CoVa monitoring system, is a large decorative necklace, with adhesive on the back of it, which patients are instructed to wear for a few minutes once a day. It sends a Bluetooth signal to either an Android tablet or a Qualcomm Life 2net Hub, which then sends the data to the cloud via cellular or WiFi. Sensors in the device track fluid levels, heart rate, respiration rate, heart rate variability, stroke volume, cardiac output, single-lead ECG, and posture.
"The tagline is we're trying to do for congestive heart failure what a glucometer [does] for diabetics," Pede said. "We've talked a lot about congestive heart failure, but we have had some interest from other industries, in terms of a low acuity necklace for stroke monitoring."
It might seem odd to have a wearable, fashion-forward device for a brief, daily check-up. But Pede says the necklace form factor both allows the company to be flexible in the development of future use cases and makes it easier to ensure consistent placement of the electrode.
The company isn't ruling out consumer use cases for the $300 device, but they intend to start out going through hospitals, for whom preventing cardiac events and their associated hospital costs could be a major driver. Perminova will submit FDA 510(k) clearance in April, after which they can start to secure more hospital partners.
Perminova is currently funded by a single angel investor according to Pede. The company is also partnering with remote patient monitoring analytics company Jointly Health.
A few years ago, the FDA cleared a similar product, Corventis's Piix sensor, for cardiac monitoring including fluid monitoring. Corventis, which was one of the first companies to be tested at the West Wireless Health Institute, has been quiet in recent years. Several other products, like the ZIO patch or Vital Connect's Health Patch, are peel and stick sensors that monitor a number of vital signs, but not fluid or stroke volume.














