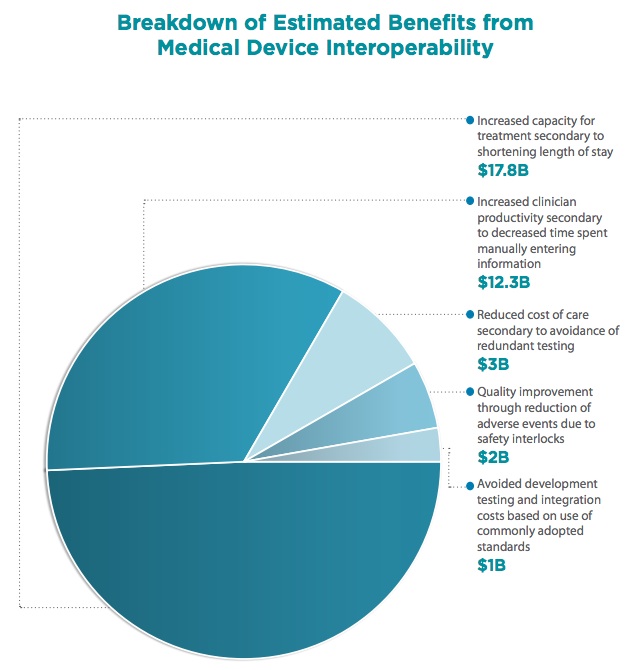
 This time last year, the Gary and Mary West Health Institute released a report calculating that medical device interoperability (via the adoption of open standards) could save the healthcare system about $36 billion. Now the Institute has teamed up with federal government groups and released a new whitepaper, based on an event held last month, detailing how interoperability -- and those savings -- could be achieved.
This time last year, the Gary and Mary West Health Institute released a report calculating that medical device interoperability (via the adoption of open standards) could save the healthcare system about $36 billion. Now the Institute has teamed up with federal government groups and released a new whitepaper, based on an event held last month, detailing how interoperability -- and those savings -- could be achieved.
"There's no doubt about it that we are asking overworked clinicians who are already trying to integrate all sorts of clinical information about how best to take care of patients with all sorts of different diseases," West Health Chief Medical and Science Officer Dr. Joseph Smith told MobiHealthNews. "And then we're going to put them into a hospital and ask them to be the conductor of an entire orchestra of complex medical equipment. We're not doing them any favors. We're not helping the physicians and we're not helping the patients."
At the Healthcare Innovation Day event, representatives from the Office of the National Coordinator of Health Information Technology and the Food and Drug Administration came out in support of open standards for medical devices.
"Interoperability is the key to facilitating health information exchange," Jodi Daniel, Director of the Office of Policy and Planning at the ONC, writes in the whitepaper. "Government has a role in setting the rules of engagement for interoperability, by supporting the establishment and oversight of a common set of behaviors, policies, and standards to enable electronic exchange of health information among participants."
Jeffrey Shuren, director of the Center for Devices and Radiological Health at the FDA, announced that the FDA would be releasing draft guidance soon on interoperability. The guidance will be non-binding, but the FDA will incentivize interoperability by making it easier for devices that met the standards to get clearance.
"FDA’s role is to provide the regulatory environment to promote interoperability," Shuren writes in the whitepaper. "The value of consensus standards is that we can say: If you conform with these standards, we’re viewing you as having met the regulatory requirements. We are not telling you that you must conform, but if you do and we have adequate assurances, that’s good enough for us. We would say that if a test bed — an independent third party — certifies you, that is good enough for us. We’re looking to provide the incentives to move toward that approach."
Smith says that there's not exactly a group opposed to medical device interoperability. Instead, progress has been slow because of misaligned incentives. While the hospitals stand to gain most of the direct savings benefit from interoperability, it's vendors and device companies that have to put in most of the work, and they get short term benefits from having proprietary systems.
"I don't think anyone's stood up and been opposed to it," he said. "It's a question of genuine versus feigned enthusiasm ... There's fairly broad acceptance of the notion that we need to get there. The 'how' is one of those things where we're trying to advocate for using market forces to get to this place even though we're here largely because of a market failure, because people find value in the proprietary systems that elevate switching costs."
Nonetheless, Smith thinks vendors will come around, just as the personal computing industry realized long ago that it's more lucrative to build a printer that worked with any computer then to sell peripherals that only work with your device.
"The market does an excellent job [of incentivizing interoperability] when the vendors are in a position to understand that the buyers have that as a user requirement," he said. "Early on, it's pretty clear that the buyers weren't asking for it because they didn't necessarily appreciate that it was even possible. Realizing there aren't significant technical hurdles to making it happen, we're now busy trying to educate the [hospital executives] that this is something you can ask for. And as that happens, we watch as the vendors step up to provide it."
For that reason, the recently-founded West Health spinoff, the Center for Medical Interoperability, is trying to achieve widespread adoption of open standards by leveraging hospital buying power, rather than relying on the hope that interoperability will make it into Meaningful Use Stage 3 guidelines.
"I think you can get there the way the Center's trying to get there, which is to organize the buying power of the largest buyers in the ecosystem, the hospitals, and have their buying be directed toward those systems that are smoothly interoperable," Smith said. "...We can be hopeful it's going to show up in Meaningful Use Stage 3, but I'm not sure it will."
In addition to the cost savings, West Health hosted the event and penned the paper to stress that interoperability has great potential to save lives and improve patient care. He cited an Institute of Medicine report saying that 100,000 people each year die of preventable medical errors.
"I don't think we're going to be able to learn as much as we need to or prevent these otherwise preventable deaths unless the data flows," he said. "It has to be the case that in order to learn you have to understand full impact and be able to review it."














