 This week the European Commission announced a consultation -- similar to a public notice from the FDA here in the US -- that asks digital health companies and others for help in identifying ways to encourage and regulate mobile health, which the Commission defines as "ways to enhance the health and wellbeing of Europeans with the use of mobile devices, such as mobile phones, tablets, patient monitoring devices and other wireless devices."
This week the European Commission announced a consultation -- similar to a public notice from the FDA here in the US -- that asks digital health companies and others for help in identifying ways to encourage and regulate mobile health, which the Commission defines as "ways to enhance the health and wellbeing of Europeans with the use of mobile devices, such as mobile phones, tablets, patient monitoring devices and other wireless devices."
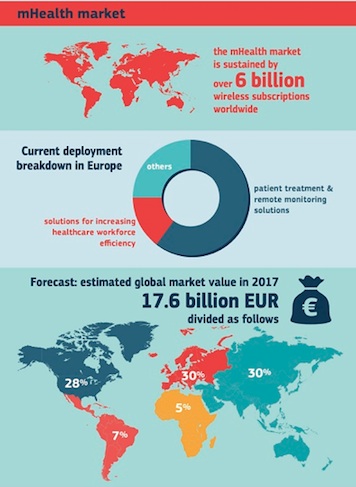
The EU is looking to move the needle on a number of issues related to mobile health in Europe, including: the safety of health apps, concerns over data misuse, lack of interoperability, and lack of understanding of legal requirements for wellness apps.
While the Commission's "green paper" on mobile health poses about a dozen specific questions to those working in digital health, a few example questions include: What safety and performance requirements should apply to lifestyle and wellbeing apps? Which security safeguards could ensure health data is safe in an mHealth context? What is the best way to promote mHealth entrepreneurship in Europe? The EU is also looking for early evidence of mobile health's impact on healthcare systems in Europe. The Commission has given a July 3, 2014 deadline for comments and it expects the consultation to inform policy decisions set to be announced sometime in 2015.
The European Commission has also funded a handful of mobile health initiatives with about €100 million already committed and about that much still available over the course of the next two years. Some of the mobile health projects that have benefitted from the EU's funding to date, include:
The Nephron Plus project received €5 million of EU funding to develop a wearable artificial kidney device, which can be remotely monitored by patients on their smartphone, and by providers.
GlucoTab, developed by the EU-funded Reaction project, is a mobile-centered system that aims to make medical information flow more freely inside hospitals. The system leverages sensors to monitor parameters like blood glucose levels, nutritional intake, administered drugs and insulin sensitivity and provides therapy advice based on the readings. Medical staff can review the data via a tablet app.
The EU-funded MobiGuide project has developed an intelligent mobile system to guide patients with chronic diseases. The research project originally focused on cardiac patients (atrial fibrillation) and women with complications during pregnancy (high blood pressure and diabetes), but the system will have a broader set of use cases. Here's how the Commission describes it: "The patient wears sensors that can monitor their biosignals (e.g. heart rate, blood pressure); these signals are transmitted to their smartphone and from there to a powerful back-end computer. The data generated is analysed by the MobiGuide decision-support tool, on the basis of patients' historical clinical data. The tool alerts the patient about actions that should be taken and asks them questions, in case additional information is needed. Then the system makes recommendations regarding lifestyle changes or contacts care providers."
Finally, the EU-funded InterStress project has created the Positive Technology App, which helps users "escape to a tropical island in 3D virtual reality where you can learn and practice effective relaxation techniques." Here's how the Commission describes it: "The aim is to reduce your stress and improve your health by self-managing and treating your stress through your smartphone or tablet. You can use compatible biosensors available in the market (e.g. a special bracelet), which enable you to control the features of the virtual environment through your heart and respiration rates; for instance, based on your heartbeat, the sensors may increase or the decrease the size of a virtual campfire or of a virtual waterfall."














