 Over the last month, several digital health companies have wound their way through the FDA clearance process. Although the details on some of these clearances are still emerging, the following is a snapshot of some of what the agency has been up to.
Over the last month, several digital health companies have wound their way through the FDA clearance process. Although the details on some of these clearances are still emerging, the following is a snapshot of some of what the agency has been up to.
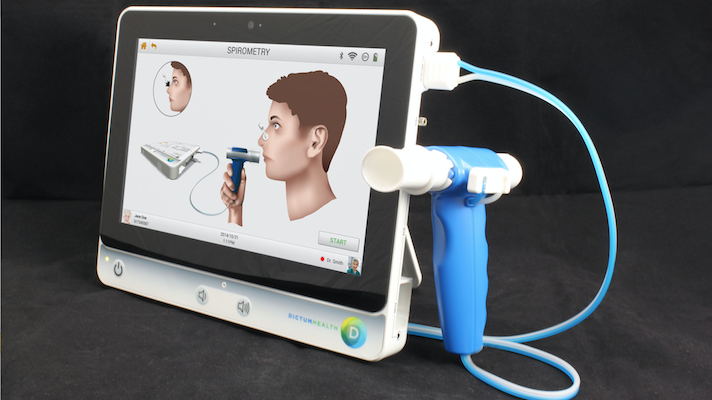
Dictum Health's telemedicine platform
Dictum Health, which makes an end-to-end telehealth platform, receieved clearance to add spirometry to the capabilities of its "virtual exam room," which can already handle Sp02, blood pressure, height and weight, temperature, and ECG. The device takes the form of a tablet that connects to a variety of health device peripherals and it is cleared for use both in hospitals and in the home. Through the tablet, patients and doctors can conduct a secure, HIPAA-compliant video visit and beam vitals data directly to the clinician.
Founded in 2013, Dictum now operates in hospitals (including an ongoing pilot at UC Davis), schools, and skilled nursing facilities. The company received its original Class 2 clearance in 2015. Spirometry will allow the device to be used in monitoring and diagnosing chronic respiratory conditions like asthma and COPD.
Philips' eCareCoordinator
Philips received an additional FDA clearance for eCareCoordinator, a telehealth application that was originally cleared in October 2014. With eCareCoordinator, clinicians can access daily summaries for each patient so that they can prioritize which patients to reach out to and what care plans they need to adjust. The app collects data from connected devices that measure health metrics including blood pressure, vital signs, and weight, but the app also gathers more subjective data from questionnaires and communication with the patient's care team.
Update: This is what the company had to say, in an email, about the latest clearance:
"The latest version of eCareCoordinator delivers personalized care plans at scale for specific populations," a spokesperson wrote. "Our software platform is designed to enable care teams to help keep patients healthier at home, which improves patient quality of life, and can lead to reduced hospital admissions, readmissions, and emergency department visits, potentially lowering cost of care. The latest release features a redesign of the User Interface based on customer feedback, as well as improved data analytics and visualization tools. This improves usability for larger scale operations, and supports health care organizations that are incorporating high acuity virtual care capabilities into their value based care programs. This is also the first version of eCareCoordinator to leverage the Philips healthcare cloud solution, the Philips HealthSuite Digital Platform, for an even more robust, secure, and scalable offering."
Osprey Medical's DyeVert System
Osprey Medical is a company focused on a product called DyeVert, which helps reduce the risk of kidney damage to patients undergoing certain surgeries, including angiograms, stent placements, balloon angioplasties. Contrast dye is injected into patients' bloodstreams to improve the effectiveness of X-Rays and CT scans. It's safe for most patients but can cause problems in patients with kidney failure. Osprey's DyeVert system uses a Bluetooth-equipped smart syringe and an associated monitor to track exactly how much dye is being used and halt the injection at a threshhold that could potentially harm the patient.
The DyeVert system recieved FDA clearance in March, but the company received another clearance for just the smart syringe component at the beginning of July. We've reached out to the company for details about the new clearance and will update if they reply.
VitalConnect's VitalWatch
VitalConnect actually recieved this FDA clearance back in April, but the summary just became available. The remote monitoring company makes a disposable peel-and-stick health sensor called VitalPatch that can measure a range of biometric data including single lead ECG, heart rate, heart rate variability, respiratory rate, skin temperature and even posture (which can detect falls). Via Bluetooth, the sensors send the data to either a connected app or hub, then onwards to a cloud-based server where it can be accessed by the user’s care providers.
The new clearance is for VitalWatch, which is described as a software user interface that can serve as a secondary, adjunct monitor for VitalConnect data. According to the company's trademark application, it seems likely that VitalWatch will be a mobile app.
The company declined to provide details, telling MobiHealthNews the clearance is part of a larger, upcoming announcement for the company, but did confirm that it is not an application for the Apple Watch.
















