 Last week Dr. Peter Budetti, Deputy Administrator for Program Integrity of the Centers for Medicare and Medicaid Services (CMS), announced the official launch of two new smartphone apps from CMS -- the agency's very first app offerings -- that aim to help physicians and others track payments and other information they receive from the industry as part of the transparency program called the Sunshine provision in the Affordable Care Act.
Last week Dr. Peter Budetti, Deputy Administrator for Program Integrity of the Centers for Medicare and Medicaid Services (CMS), announced the official launch of two new smartphone apps from CMS -- the agency's very first app offerings -- that aim to help physicians and others track payments and other information they receive from the industry as part of the transparency program called the Sunshine provision in the Affordable Care Act.
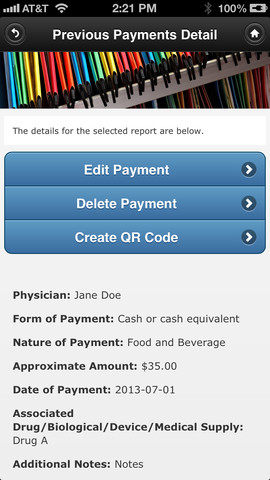
The apps, which are available for iOS and Android users, are for tracking and storage only -- not for reporting or sending data to CMS. The agency created an app for physicians and one for companies that work with them.
"The Open Payments law is designed to improve transparency to sustain patient care and trust as the highest priority by allowing the public to be informed about financial relationships that could influence the drugs, devices, biological, or medical supplies used in health care delivery," Budetti explained in a CMS blog post. "To facilitate transparency and to keep patient care and trust as the highest priority, CMS has developed these apps to help physicians and others managed their oversight of information about their financial relationships that companies will report to Open Payments. We developed these apps to reduce the reporting burden by providing tools to simplify the tracking process and help improve data accuracy reporting for the industry (manufacturers and GPOs)."
The apps can also help physicians and the companies they have financial ties with to compare notes and ensure they are both reporting the same information. Budetti laid out an example use case for the app in his blog post:
"The physician can use the 'Read Quick Response (QR) Code' functionality that allows the manufacturer to create a record of the interaction and transfer it to the physician for her review. Additionally, to help ensure the drug manufacturer correctly attributes the consulting fee to her, she can share her profile information with the drug company using the “Create QR Code” function. Months later, when she is reviewing the data the drug manufacturer reported to CMS, and before the data is made public, she can retrieve her original record from her mobile device and do a comparison to confirm that the information is correct. If she believes the information that the manufacturer submitted to CMS about a particular interaction is not accurate, she can work with the manufacturer on correcting the information prior to publication."
Early last year CMS announced plans to leverage the White House-backed Text4Baby program to increase enrollment in Medicaid and CHIP.
More from Budetti on the new apps here.
















