 There are 229 dermatology-focused medical apps in the Apple, Android, Blackberry, Nokia Windows app stores, according to a study recently published in the Journal of the American Medical Association Dermatology. More than half of the apps are patient-facing (51 percent), with 41 percent targeted at health care providers and 8 percent aimed at both groups.
There are 229 dermatology-focused medical apps in the Apple, Android, Blackberry, Nokia Windows app stores, according to a study recently published in the Journal of the American Medical Association Dermatology. More than half of the apps are patient-facing (51 percent), with 41 percent targeted at health care providers and 8 percent aimed at both groups.
The authors excluded certain apps form their count, such as advertisements for products or practices, apps for cosmetics or entertainment, or claims to cure skin diseases. The latter category evokes two apps that were removed by the FDA a few years ago, which claimed to cure acne using colored lights. Researchers didn't download or evaluate the apps; they merely drew data from the public descriptions in the app store.
Just over half of the dermatology apps were free of charge (51 percent) while paid apps ranged from 99 cents to $139.99. The high end was mostly medical textbook apps, however, and the median was $2.99.
The largest category of apps was reference apps, which included both medical textbooks and informational guides for the general public. These made up 27 percent of the apps found.
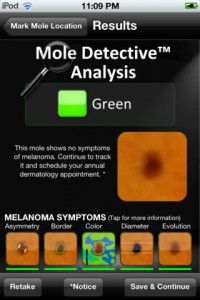
The next largest group, making up 18 percent, was self-surveillance or diagnosis apps, which allow users to take pictures of their moles or skin lesions, track them and, in some cases, analyze those pictures to give the user a risk assessment. These have been among some of the most controversial apps in not just dermatology, but medicine in general. Apps like this are a common talking point in discussions of mobile health regulation, including being mentioned in the Congressional Hearings on health app regulation as a potentially dangerous app.
Nearly as many apps (17 percent) were characterized as disease guides -- apps that are focused on a particular disease like acne, rosacea, psoriasis, or eczema. Other categories included sunscreen recommendation apps (8 percent), educational aids (9 percent), dosing calculators for dermatology medications (5 percent), dermatology journal and conference apps (5 percent), and teledermatology apps (3 percent).
The study was notably completed and written before the FDA published its final guidance on mobile medical apps. Those guidelines place some dermatology apps, like skin lesion trackers, in the category of devices over which it will exercise its enforcement discretion, meaning they will choose not to regulate them. Apps that provide diagnosis, however, will likely require 510(k) clearance.




















