 Boston and San Francisco-based Pear Therapeutics, not to be confused with Pear Sports, has raised an undisclosed sum in a round led by 5AM Ventures.
Boston and San Francisco-based Pear Therapeutics, not to be confused with Pear Sports, has raised an undisclosed sum in a round led by 5AM Ventures.
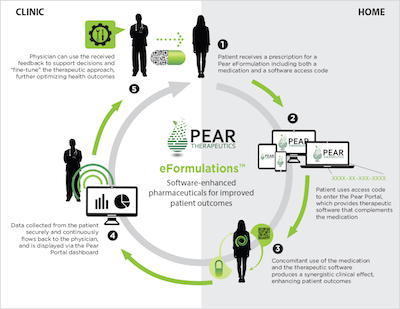
The company has developed a product called eFormulations that combines medication for a specific condition with a digital therapy program. The eFormulations product will be prescribed by physicians to their patients.
Depending on the patient's condition, the software component of the offering could include games, tracking tools, and other engagement features.
"What is fundamentally different about the Pear story is that we are pushing intervention content, which has the ability to enhance the efficacy of the therapy beyond just compliance," Pear Therapeutics CEO Corey McCann told MobiHealthNews. "I think the nice example to tee up here is the treatment of depression. If I'm a patient with a major depressive disorder, I will get a combination of an anti-depressant plus some form of cognitive behavioral therapy (CBT) that’s been... recapitulated in a digital form. In some cases a digital CBT actually works better than an in-office CBT. There's a huge opportunity to create a multi-modal therapeutic package, which is an anti-depressant plus CBT software. So that’s one example of how we can bring these two things together to simultaneously impact brain chemistry and to make this better than the pill alone."
The program will also collect data from the patients to send to a physician. Each therapeutic package collects different data sets because the products are addressing different conditions, but generally Pear will aggregate patient input data, performance data collected from a patient's gameplay, and passive monitoring data, like voice data and accelerometer data, which is collected with the patient's consent. The passive monitoring data will be used to quantify and qualify a patient's mood.
Pear Therapeutics plans to first release products for addiction and PTSD, followed by depression, general anxiety, and sleep. After that, McCann said the company has another five products they plan to push through the regulatory process. None have been cleared by the FDA yet. Pear plans on a 510(k) clearance for the software, but the company will then go after a 505(b)(2) clearance for combo products involving both software and a drug.
"For a drug-software combination, we are creating a drug-device combination therapeutic, much in the same way that one develops a respiratory combination by taking a fixed dose inhaler plus a dry powder," McCann said. "The inhaler is the device, the dry powder is the drug. We are doing the same thing here, it’s just that the device happens to be software and the drug is the drug. So in the same way that combination products in the respiratory space are prescribed and reimbursed as a branded pharmaceutical product, we are doing the same thing for drug-software combinations."




















